Table of Contents
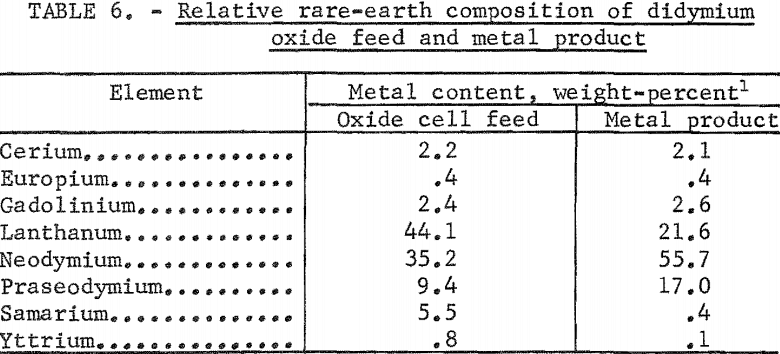
Electrowinning Rare Earth Elements like Neodymium, praseodymium, and didymium, were (obviously) electrowon from their oxides dissolved in fluoride melts. The use of a thermal gradient cell resulted in recovery of nodules of well-coalesced metal with a purity of 99.9 percent. Current efficiencies of up to 83 percent were obtained. Shallow immersion of fluted anodes allowed carbon oxide gases to escape readily from the electrolyte and resulted in low carbon and oxygen impurities in the metal product. Electrowon didymium metal was enriched in neodymium and praseodymium and impoverished in lanthanum, samarium, and yttrium compared with the oxide cell feed.
For most of the 20th century, neodymium, praseodymium, and didymium (the cerium-free mixture of light rare-earth elements) have been won by electrolysis of the fused chlorides. As early as 1904, Muthmann and Weiss prepared neodymium metal from neodymium chloride (NdCl3). Much information on metal electrowinning on a laboratory scale has been published. For example, Trombe used 25 grams of electrolyte composed of 60 percent NdCl3, 35 per-cent potassium chloride (KCl), and 5 percent calcium fluoride (CaF2). Neodymium and praseodymium have also been produced by reducing the chlorides or fluorides with calcium or lithium. Recently, neodymium metal was prepared on a laboratory scale by calcium reduction of the fluoride and was purified by vacuum distillation; the purified metal was reported to contain 0.015 percent oxygen and up to 0.05 percent tantalum as major impurities.
High-purity cerium and lanthanum were electrowon, as liquid metals, from their oxides in fluoride melts. The cells were simple in design with graphite as the major structural material. Electrowinning the metals in the liquid state minimized the formation of high-surface-area products and facilitated metal-electrolyte separation. Collecting the metal nodules on a skull of frozen electrolyte prevented their contamination by the graphite crucible. The success of this technique prompted the present study for preparing neodymium, praseodymium, and didymium. The thermodynamic stability, conductivity, and low vapor pressure of NdF3, PrF3, and LiF, as well as their solubility for rare-earth oxides, were determining factors in the selection of these materials for electrolyte components.
Equipment
Neodymium, praseodymium, didymium metals, and the electrolyte constituents react with air at cell operating temperatures; therefore, the electrowinning cell was enclosed in a gastight steel chamber. The essential features of the chamber are diagrammatically illustrated in figure 1. Glove and viewports enabled the operator to add electrolyte and feed and to adjust the electrodes during electrowinning experiments. Heat lost from the exposed
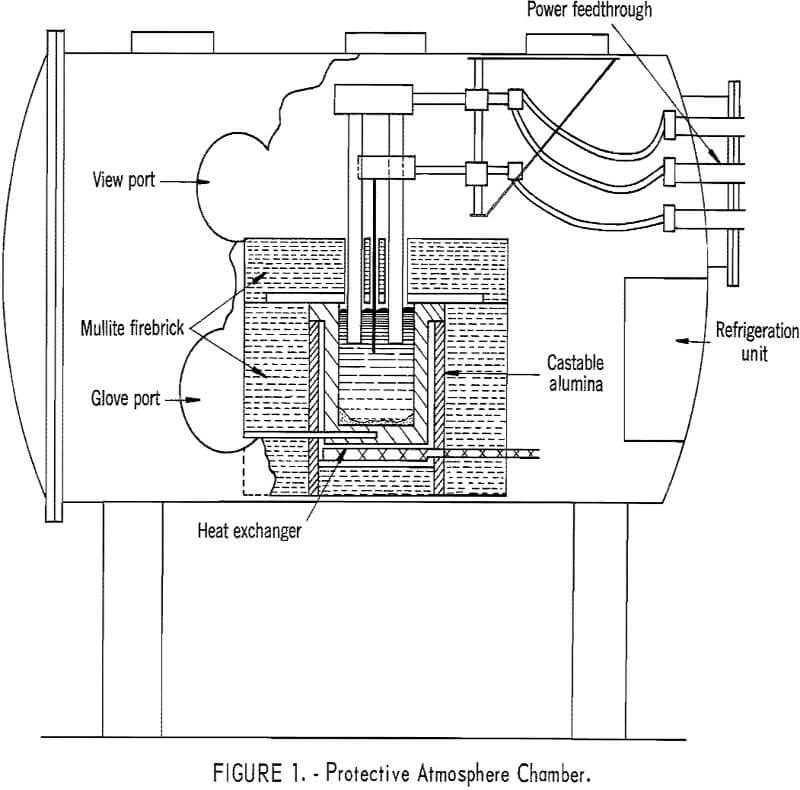
electrodes and molten bath would have raised the atmospheric temperature within the chamber above 100° C. However, a refrigeration unit installed in the chamber maintained the temperature at approximately 50° C, and manual operations were performed without discomfort.
Power for electrolytic reduction was supplied by a 0- to 40-volt, 0- to 300-ampere silicon rectifier. A single-phase, continuous control, 0- to 750-ampere alternating-current arc welding unit provided auxiliary power for maintaining the bath temperature. Bath and crucible bottom temperatures were measured with platinum, platinum-rhodium thermocouples.
Mullite firebrick was used for the furnace, shell structure and primary insulation. The inside of the furnace adjacent to the cell was lined with castable alumina. The cell cover over the crucible was lined with graphite to protect the brick from corrosive fluoride fumes and electrolyte splash.
In all electrowinning experiments, the crucibles holding the molten electrolyte were AGX-grade graphite machined from 6-inch electrode stock to an inside diameter of 4 to 5 inches and a depth of 6 inches. The cathode was a tungsten rod 0.2 inch in diameter, and the anodes were graphite rods 0.75 or 1 inch in diameter.
Cell Design and Operation
Experience in electrowinning rare-earth metals in these laboratories has indicated that a relationship exists between the temperature of the metal collection zone and the recovery and purity of the product. For example, optimum recovery of the highest purity lanthanum was achieved by electrodepositing the liquid metal at 950° C and collecting it in the form of nodules at 700° C on a frozen electrolyte skull. Observations such as this suggested the need for a thermal gradient cell in neodymium and praseodymium electrowinning studies. The requisite conditions for operating the thermal gradient cell were obtained by internally heating the electrolyte and simultaneously cooling the bottom of the crucible.
Cells were internally heated by either of two methods. In the first, supplemental heat was obtained by applying alternating current between the two anodes. The design of this cell is shown in figure 2. In the second, heat was obtained by applying alternating current to two 0.4-inch-diameter tungsten heater rods immersed in the bath. The graphite crucible served as the anode in these experiments.
Before starting electrowinning experiments, an inert atmosphere was obtained in the cell chamber by pumping and backfilling with helium gas. The fluoride electrolyte powder was charged to the cell, and meltdown was initiated by short circuiting alternating current between the anodes and crucible. After a molten pool of electrolyte was obtained, the anodes were raised to electrolysis position; additional electrolyte powder was charged, and heating continued until the required cell temperature was attained. Electrolysis was then started, and the appropriate oxide (neodymium, praseodymium, or didymium) was intermittently fed to the cell. The rate of feed was related to the

direct current; oxide was added at about the same rate as the expected CO2 and CO evolution from the anodes.
A controlled flow of air or helium through a copper coil located beneath the crucible cooled the bottom of the crucible and maintained the desired temperature gradient between the zones of metal deposition and collection. A thermocouple located in the crucible bottom was used to measure the temperature of the metal collection zone.
Temperature measurements of the bath were made intermittently with an immersion thermocouple. Gas samples were taken from above the electrolyte and analyzed for carbon oxides and fluorocarbons by the Orsat method and infrared spectrophotometry.
After completion of an experiment, the electrolyte was allowed to cool to ambient temperature before the crucible was removed from the chamber. The contents of the cell were then crushed, and the metal nodules were recovered and sampled for analysis. Crushed electrolytes were used as baths in subsequent electrowinning experiments.
Electrowinning Neodymium
The electrolyte constituents were reagent-grade lithium fluoride (LiF) and 99.9 percent neodymium fluoride (NdF3). The neodymium oxide (Nd2O3) cell feed contained <0.1 percent total impurities. The three bath compositions studied were 90 weight-percent NdF3, 10 weight-percent LiF; 80 weight-percent NdF3, 20 weight-percent LiF; and 74 weight-percent NdF3, 26 weight-percent LiF, The solubility of Nd2O3 in these baths was determined to be of the order of 2 percent by a method previously described. The amount of Nd2O3 fed to the cell and data from analysis of frozen baths for oxygen indicated an oxide utilization of about 60 percent.
Little difference was observed in the operating characteristics of cells containing any one of the three baths. However, the frozen bath skull for a metal collection hearth was most easily maintained with the 90 weight-percent NdF3, 10 weight-percent LiF melt which has a solidus point of 780° C. In frozen baths examined after electrolysis the skull, from ½ to 2 inches thick, was a deeper purple and denser than the upper part of the bath. Analytical data indicated that excess Nd2O3 had settled to the bottom and had become part of the skull.
The data in table 1 were obtained from a neodymium electrowinning experiment in which the bath was heated by applying alternating current to the anodes. During electrolysis, a combined alternating and direct current power of approximately 6.0 kilowatts was required to maintain the temperature of the metal deposition zone at about 1,100° C. The temperature of the metal collection zone was held at 750° C by cooling the bottom of the crucible.
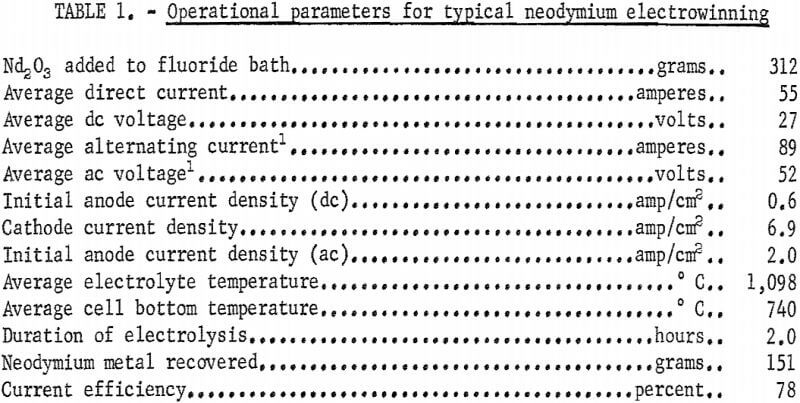
Analysis of the atmosphere, in the cell chamber after 1 hour of electrolysis showed 7 percent CO, 3 percent CO2, and 0.5 percent CF4; the rest was helium. The CF4 indicated that some electrolytic reduction of NdF3 or LiF had occurred.
When the bath was heated by applying alternating current to two tungsten rods, a third tungsten rod served as the cathode, and the graphite crucible was the anode. No electrolyte skull was maintained. In a typical run, the average direct current was 109 amperes at 7 volts for 1 hour of electrolysis. Current efficiency was 55 percent. The direct current amperage obtained was higher than in previous experiments and resulted in a higher rate of metal production. A disadvantage of using the crucible for the anode was increased carbon impurity in the metal product. For example, neodymium prepared in this cell contained about five times as much carbon as metal prepared in the cell with graphite rod anodes.
In cells with either anode rods or an anodic crucible, a high degree of coalescence of the metal product was attained. In most experiments, over 80 percent of the neodymium prepared was in individual nodules weighing from 50 to 200 grams each.
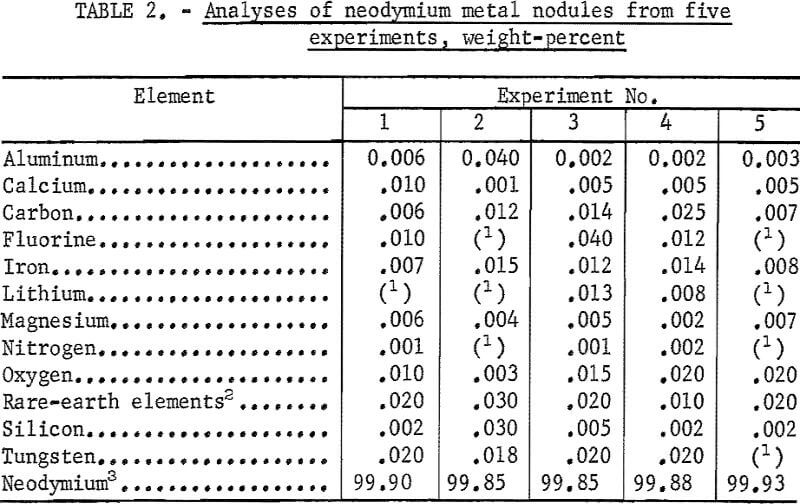
Table 2 contains the analytical data for neodymium metal nodules from five experiments in which the anodes were two graphite rods. Carbon and oxygen content was determined by inert gas fusion techniques. Spectrographs and ion exchange polarographic techniques were used to determine metallic impurities. Individual nodules contained from 0.006 to 0.025 percent carbon and from 0.003 to 0.02 percent oxygen. Tungsten, the major metallic impurity, was <0.025 percent. Nodules ranged in fluorine content from 0.01 to 0.04 percent ; microscopic examination showed that some of the nodules contained small amounts of interspersed electrolyte, a possible source of the fluorine contamination.
To demonstrate the advantages of and the need for a thermal gradient cell, experiments were made on neodymium preparation. Cell arrangement, electrolyte, feed, and operating conditions were as previously described (fig. 2), except that the cooling apparatus was not activated. The metal was collected on a tungsten hearth at 1,100° C. Recovery was poor. Current efficiency was <40 percent (about one-half that obtained in the temperature gradient cell experiments). The concentration of carbon in the metal was >0,08 percent (4 to 10 times greater than in the temperature gradient cell experiments). Therefore, electrowinning of neodymium, praseodymium, and didymium was done only in thermal gradient cells to obtain maximum yield and purity.
Electrowinning Praseodymium
High-purity praseodymium metal was won from both Pr6O11 and Pr2O3 dissolved in PrF3-LiF melts. The electrolyte constituents and cell feed were reagent-grade materials. The melt composition was 60 weight-percent PrF3, and 40 weight-percent LiF; it had a solidus point of 760° C. However, two problems were found in winning praseodymium that were not encountered in the neodymium cell.
The first problem was the severe corrosion of the graphite anodes and tungsten cathode at the surface of the molten bath when Pr6O11 was used as cell feed. This problem was similar to one encountered in electrowinning cerium from CeO2. In the cerium cell, excessive burning of the graphite anodes was attributed to the high CO2-to-CO ratio produced in the anode reaction and was overcome by using Ce2O3 instead of CeO2. Experiments were made in the praseodymium cell using Pr2O3 as the feed material. Examination of the electrodes after the runs revealed that corrosion at the melt surface was eliminated by feeding the sesquioxide. Because praseodymium sesquioxide is not commercially available, it was necessary to prepare it.
The carbon reduction technique for preparing Ce2O3 was applied to the preparation of Pr2O3. A charge of 50 grams of Pr6O11 was mixed with the stoichiometric amount of minus 200-mesh graphite; the mixture was placed in platinum crucibles in a tube furnace and flushed with helium. The furnace was heated to 1,100° C and maintained at temperature for 3 hours. Then the furnace was turned off and purged with helium until the charge had cooled to room temperature. The product was olive green in color and contained <0.02 percent carbon. X-ray diffraction and oxygen analysis identified the product as Pr2O3.
The second problem was the dissolution of the tungsten rods that supplied supplemental heat to the electrolyte. This reaction was attributed to an oxidizing species present in the electrolyte, possibly the tetravalent ion of praseodymium. Previous investigators have obtained the tetravalent ion in double salts with alkali fluorides; for example, the compounds Na2PrF6 and KgPrF6 have been prepared and identified. To solve the tungsten corrosion problem, it was necessary to eliminate the tungsten rods and to supply auxiliary power through graphite anodes.
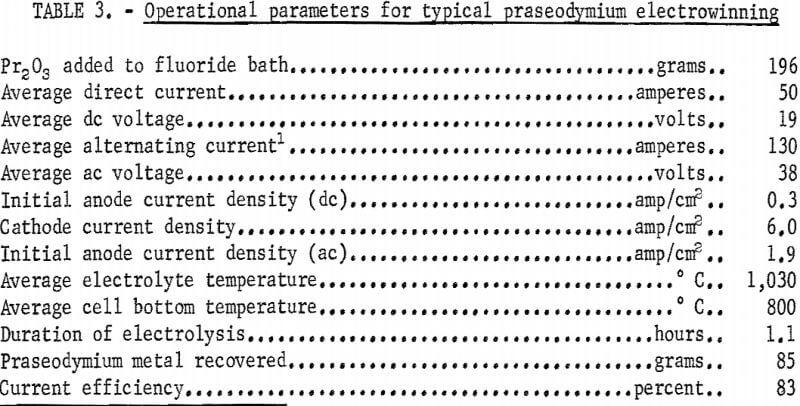
These anodes were fluted in order to increase their surface area, thereby decreasing anode effect. Twice as much current was passed through the praseodymium as through the neodymium cell for a given anode immersion depth; with the fluted anodes the desired direct current amperage could be maintained with the anodes immersed only 1 inch. This shallow immersion of the anodes allowed carbon oxide gases to escape readily from the bath and resulted in metal containing as little as 0.01 percent total carbon and oxygen impurity. Data from a typical electrowinning experiment using two fluted anodes and Pr2O3 as cell feed are given in table 3. Analysis of cell gases after 1 hour of electrolysis showed 6.5 percent CO, 1 percent CO2, and 1 percent CF4.
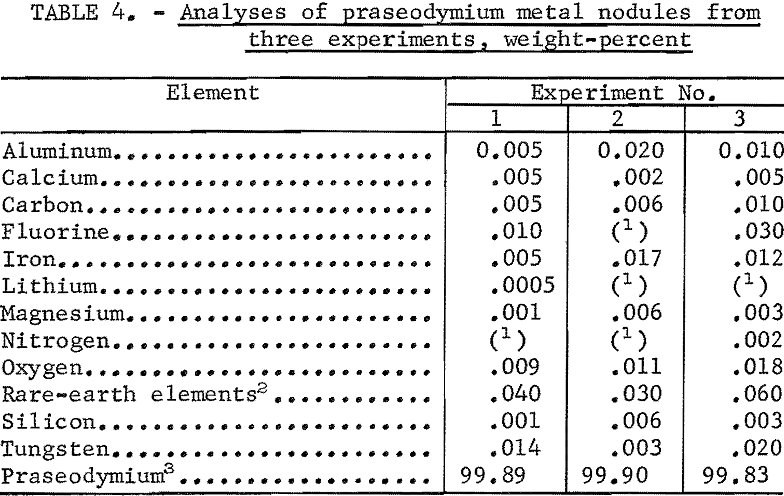
Coalescence of the praseodymium product was generally good. In most experiments, metal nodules weighing from 5 to 120 grams were recovered from the frozen baths, and nodules weighing over 40 grams represented more than 80 percent of the metal recovered. Table 4 gives analytical data of praseodymium metal nodules from three experiments.
Electrowinning Didymium
Misch metal electrowon from rare-earth chlorides has been found to contain proportionately greater concentrations of neodymium and praseodymium than the electrolyte. To determine if disproportionate deposition would also occur in the electrolytic reduction of a mixed ceric-group oxide, a study was made using a commercial didymium oxide as feed to a didymium fluoride-lithium fluoride melt. The fluoride was prepared from the oxide; therefore, the electrolyte had the same relative rare-earth composition as the oxide feed. The method of fluorination was similar to one developed by the Bureau of Mines for preparing cerous fluoride and described in a previous report.
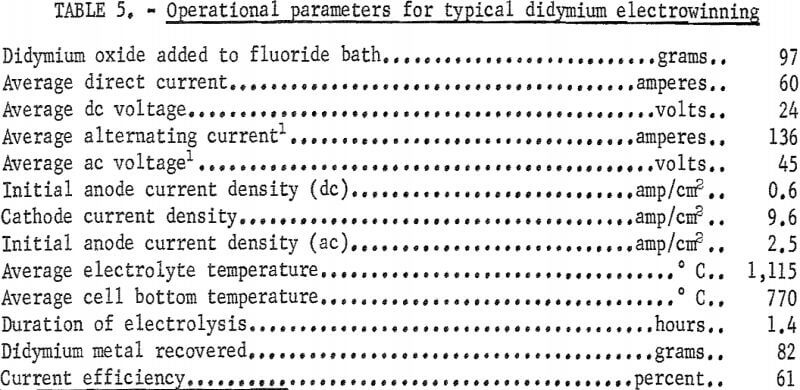
The operational data in table 5 were obtained from a typical experiment in which the alternating current was applied to two fluted anodes. Approximately 7.6 kilowatts of combined alternating and direct current power maintained the temperature of the metal deposition zone at about 1,100° C. The temperature of the metal collection zone was approximately 800° C. The metal collected was in the form of well-coalesced nodules, some weighing as much as 80 grams. Analysis of didymium nodules for carbon and oxygen gave results comparable to those obtained for praseodymium nodules (table 4).
The relative rare-earth compositions of cell feed and metal product are compared in table 6. The data show approximately twofold enrichment in neodymium and praseodymium. The lanthanum content of the metal product is about one-half that of the oxide feed; the yttrium content, one-eighth; and the samarium content, one-fourteenth. A comparison of values for free energy of formation of the oxides alone does not explain this preferential deposition. Differences in the solubility of the oxides in the fluoride melt and in the formation of complex ions may play important roles. Investigators of metallothermic reduction techniques for preparing rare-earth metals have noted the stability of bivalent samarium. This stability may account for the samarium ion remaining in the electrolyte.