Table of Contents
A molten-salt electrorefining process was developed which provides a practical way for reclaiming vanadium from scrap generated in the processing of ductile vanadium into bar, sheet, wire, and other shapes. Refining produced extremely ductile vanadium with a purity range of 99.93 to 99.95 percent. The process was particularly effective in reducing the interstitial elements, carbon, nitrogen, and oxygen, and some of the metallic impurities in the scrap.
The objective of this investigation was to develop a molten-salt electrorefining process for converting vanadium scrap into ductile vanadium as part of the Bureau of Mines research program on the extraction and utilization of domestic vanadium resources.
Ductile vanadium has been prepared by reducing vanadium pentoxide with calcium with aluminum followed by zone refining; decomposing vanadium iodide, reducing vanadium chlorides metallothermally, and refining commercial vanadium electrolytically in fused-salt media. Vanadium scrap is generated during the processing of ductile vanadium into bar, sheet, wire, and other shapes.
Reclaiming the vanadium from vanadium scrap had not been previously investigated. The presence of oxygen and nitrogen in the scrap made recovery of ductile vanadium by remelting impractical. Fused-salt electrorefining in an inert atmosphere is an effective process for eliminating nitrogen and oxygen in vanadium and producing a ductile product. Therefore, the Bureau investigated the use of the same techniques to convert vanadium scrap into ductile metal.
Inert-atmosphere control in the refining process was necessary for two reasons: (1) The affinity of vanadium for oxygen and nitrogen and (2) the oxidation of the vanadium dichloride content of the electrolyte by air to form volatile oxychlorides. This report describes the development of the refining process for preparing high-purity ductile vanadium from scrap.
Description of Equipment and Procedure
Electrolytic Cell
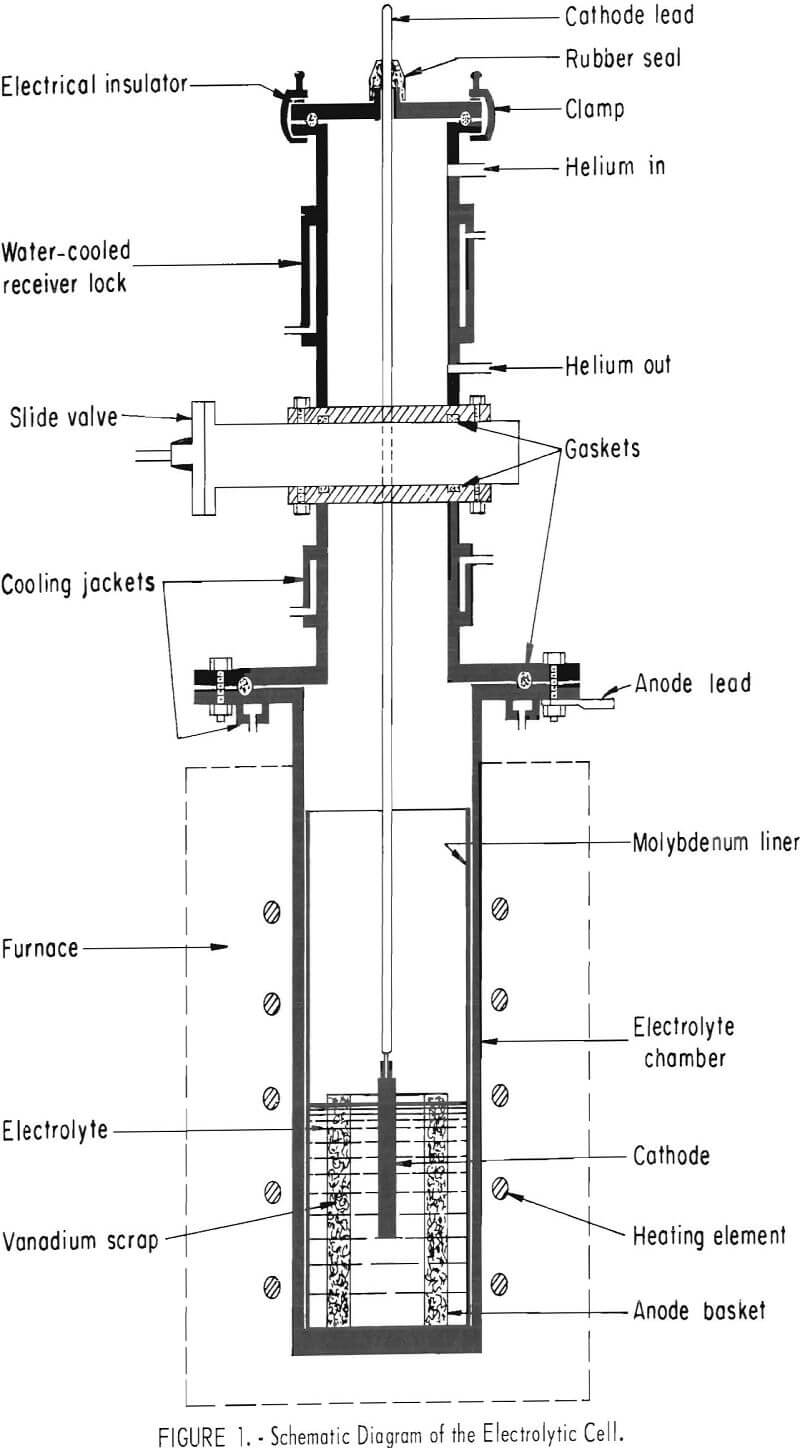
Figure 1 is a diagram of an inert-atmosphere cell used in the investigation. The electrolyte chamber was constructed of Hastalloy N, ½ inch thick, 12 inches in inside diameter, and 36 inches high. The top of the chamber was equipped with a water-cooled, mild-steel flange, A 12-inch-diameter, 24-inch-high, 13-gage molybdenum liner was used as the electrolyte container in the chamber. A water-cooled slide valve was provided for making a gas tight seal between the electrolyte compartment and the receiver lock. The lock was equipped with connections for helium and was water jacketed to accelerate cooling of the cathode deposit. The cell was evacuated through the helium out-gas connection. A metal cover with cathode rod fitting and sight glasses for observation inside the cell was clamped on the top of the lock. Seals between various parts of the cell were made with flat or O-ring rubber gaskets, protected where necessary by cooling jackets.
A 25-kilowatt resistance furnace supplied heat for maintaining the desired molten temperature of the electrolyte. The current used for electrolysis was supplied by a 12-volt, 100-amp, full-wave selenium rectifier with variable output.
Materials
The scrap used was supplied by the Vanadium Corporation of America and was generated during fabrication of ductile vanadium. The average vanadium content of the scrap was 99.4 percent. The chief impurity elements in the scrap were oxygen, nitrogen, and carbon which totaled 0.3 percent. The scrap also contained molybdenum, iron, chromium, copper, cobalt, and nickel as metallic impurities. The hardness of the scrap ranged from 95 to 110 Rockwell B, Figure 2 shows the unsegretated vanadium scrap.
An electrolyte composed of potassium chloride (KCl), lithium chloride (LiCl), and vanadium dichloride (VCl2) was chosen for the investigation. This electrolyte had previously been used in refining 90 percent commercial vanadium to produce good quality ductile vanadium with efficient cell operation.
Operating Procedure
The assembled cell was first evacuated to a pressure of 0.1 mm mercury at 25° C and then filled with helium to 770 mm mercury. The cell was leak checked with a helium-leak detector, type 20-120, Consolidated Electrodynamic Corp. The procedure was repeated after the electrolyte chamber was heated to 700° C. If helium was not detected at these two temperatures, the cell was ready for operation. A salt mixture composed of 56 weight-percent KCl and 44 weight-percent LiCl was charged into the cell. It was first dried at 250° C under vacuum for 12 hours and then melted at 400° C under a helium atmosphere. Vanadium dichloride was added by chlorinating vanadium contained in a graphite tube immersed in the molten salt at 760° C. Details of the chlorination procedure have been presented in a previous paper. The vanadium dichloride dissolved in the molten salt to form the KCl-LiCl-VCl2 electrolyte. The electrolyte was maintained at an operating temperature of 615° C through all the refining tests.
The scrap was passed through an electromagnetic separator with a field strength of approximately 15,000 gauss to remove tramp iron and other magnetic impurities introduced in the processing of the ductile vanadium. The magnetic fraction amounted to less than 1 percent of the scrap and was discarded. The scrap was then segregated manually into three types to facilitate processing: (1) Heavy metal consisting of bars, ingots, tubes, and pipes, (2) light metal consisting of foil, springs, wire, and turnings, and (3) offgrade calcium- reduced metal granules. The heavy scrap amounted to 67 percent of the non-magnetic fraction, the light scrap to 17 percent, and the metal granules to 16 percent.
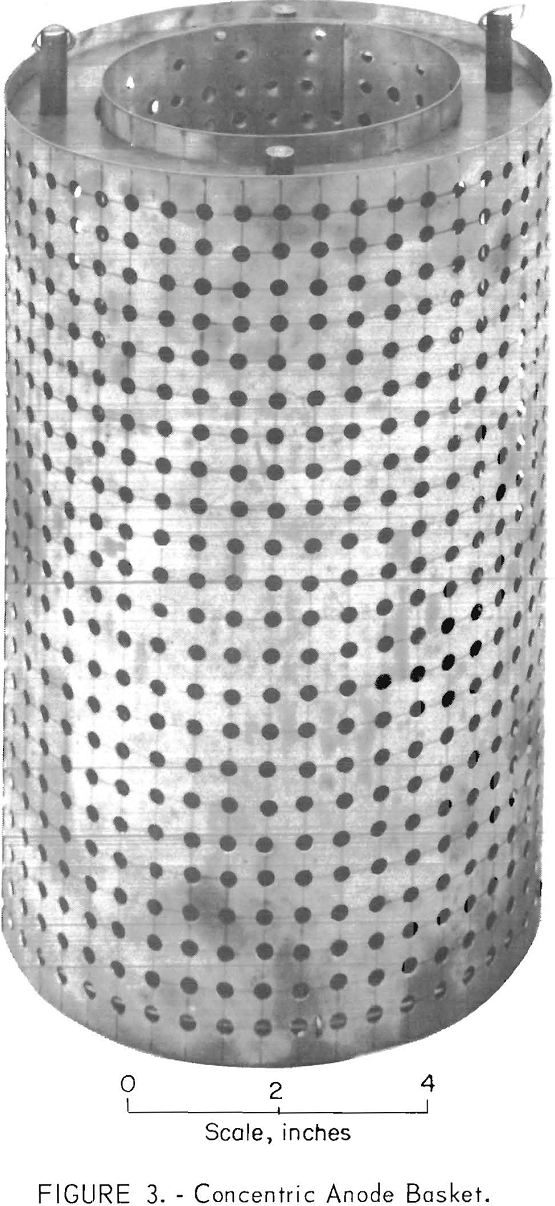
Each fraction of the scrap was leached in dilute hydrochloric acid (1:10) to remove the surface oxidation, washed with water, rinsed with acetone, and dried. The scrap to be refined was loaded into a retrievable molybdenum basket which was placed on the bottom of the cell. The basket, shown in figure 3, had a capacity of about 25 pounds of anode material which varied according to the type of scrap. The heavy scrap and the granular scrap were suitable for direct loading into the anode basket; the light scrap was compacted before use. The anode connection to the scrap metal in the anode basket was made through the electrolyte chamber and molybdenum liner. The cathode connection was made to the cathode lead rod.
Molybdenum rods 12 inches in length and 7/16, ½, and ¾ inch in diameter were used as cathodes. The choice of a cathode size depended on the electrolyte depth and the desired cathode current density for electrolysis. The cathode was immersed in the electrolyte at depths ranging from 4 to 9 inches.
In this report, a test consists of one deposit, and a series is a consecutive group of tests with a single anode addition. In a series,

electrolysis was continuous except for the time necessary to remove the cathode deposit and insert a new cathode.
When a test was completed, the cathode deposit was raised from the electrolyte until the electrical circuit was broken and adhering salt was drained from the deposit for 10 minutes. The deposit was cooled to below room temperature (less than 25° C) in the receiver lock and removed after the slide valve was sealed. The metal was stripped from the cathode, leached in dilute hydrochloric acid (1:20) to remove the entrained electrolyte, washed with water until the wash solutions were free of chlorides, rinsed with acetone, and dried.
At the end of each series, the anode basket was removed. It contained anode residue or sludge in addition to any unattacked scrap material which remained after dissolution of the scrap. The un-attacked scrap was recovered, and the sludge was discarded.
In the refining experiments, initial cathode current densities ranging from 200 to 400 amp/ft² were used in each series. The cathode current density reported for each series was the averaged value at the start of each test. The potential reported was the average of the initial potential value for each test. A constant current was maintained for each test.
Experimental Investigation
The process of electrorefining vanadium in molten electrolytes containing vanadium dichloride is based on two mechanisms: (1) The reduction of vanadium dichloride to vanadium at the cathode site and the deposition of metal on the cathode, and (2) the oxidation and dissolution of vanadium from the anode material as vanadium dichloride which replenishes the vanadium content of the electrolyte at the same rate that is depleted at the cathode. Reduction of an impurity element by electrorefining will occur if the element is less easily oxidized than the vanadium at the anode. Elements that are not dissolved remain as sludge. Success in the electrorefining process is dependent on the selective solution of vanadium into the electrolyte.
The chief contaminants which contribute significantly to the brittleness of vanadium are oxygen, nitrogen, and carbon. The prime objective in refining vanadium scrap was to reduce these impurity elements and convert the scrap into ductile vanadium. In addition to reducing the major embrittling elements, reduction of the other impurities present in the scrap was sought.
Heavy Scrap
Refining
The heavy scrap was refined in four separate series. Table 1 shows the operating data. Seventy-three pounds of KCl-LiCl-VCl2 electrolyte were initially prepared. This gave a molten-salt depth of approximately 11 inches. Prior to series 3, addition of the eutectic composition of KCl-LiCl was made to restore the original depth of the molten electrolyte. The decrease in the vanadium dichloride concentration as a result of salt addition did not change the effectiveness of the operation.
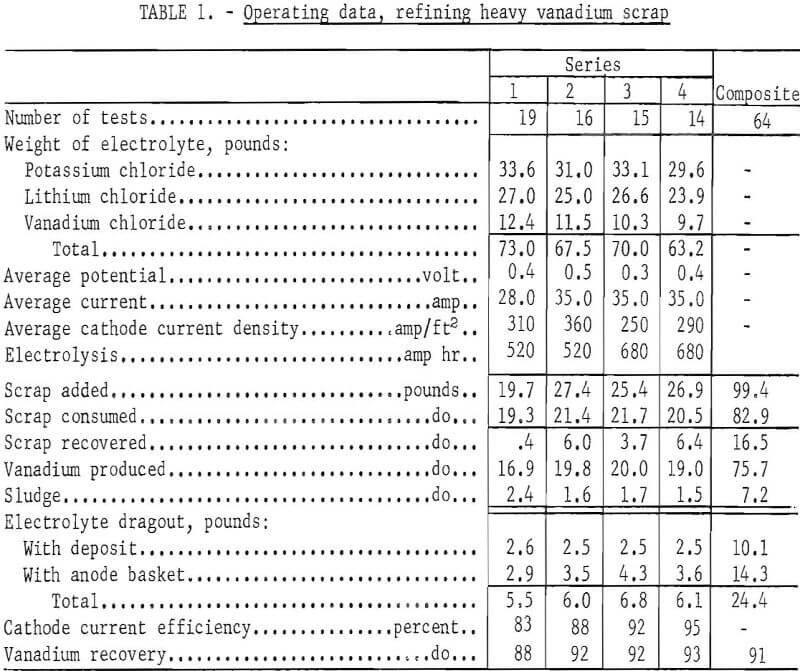
Except in series 1, over a pound of coarse dendritic vanadium crystals were obtained in each deposit. Figure 4 shows a typical unleached deposit. The average

metal-to-electrolyte dragout ratio in the deposit was 8.0 to 1.0.
In 64 tests 75.7 pounds of refined vanadium were reclaimed from 82.9 pounds of consumed scrap, and another 16.5 pounds of the 99.4 pounds of scrap originally added were recovered from the anode basket and could be reused as cell feed; 7.2 pounds of scrap were lost as sludge, which either remained in the cell bottom or was washed out as waste during the process of anode recovery. Based on the amount of vanadium produced and the scrap consumed, a vanadium recovery of 91 percent was obtained from the cell feed.
Quality of Product
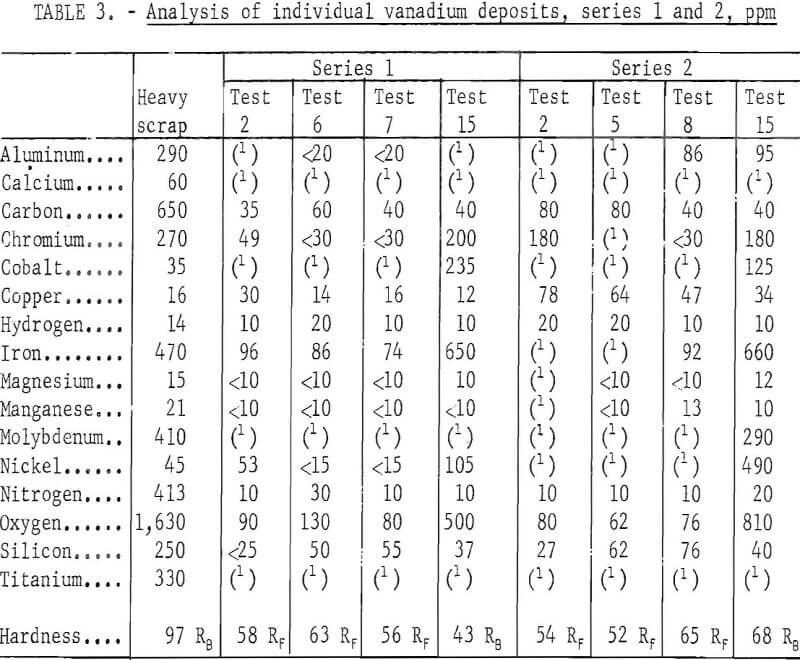
Table 2 compares the quality of the heavy scrap and the refined vanadium. The metallic impurities in the vanadium were determined by emission spectrography. Metallic elements not shown in the table were not detected by emission spectrography. Nitrogen and carbon in the vanadium were determined by Kjeldahl extraction and by conductometric combustion methods, respectively. Hardness of the metal was determined on a 30-g sample of crystals which was arc-melted into an ingot. Samples from this ingot were analysed for oxygen and hydrogen by vacuum fusion extraction. The data shown in table 2 represent the analyses of the composited re-fined vanadium from all tests and the scrap.
All impurity elements except cobalt, copper, and nickel were substantially reduced in the refined products. The chief contaminants with concentration over 100 ppm were iron and oxygen. Cobalt and nickel contents in the refined vanadium remained about the same as in the scrap. Copper was higher in the product than in the scrap. The refined vanadium contained 715 ppm impurity elements, compared to 4,919 ppm impurity elements in the scrap. The product was ductile, as indicated by its low hardness value.
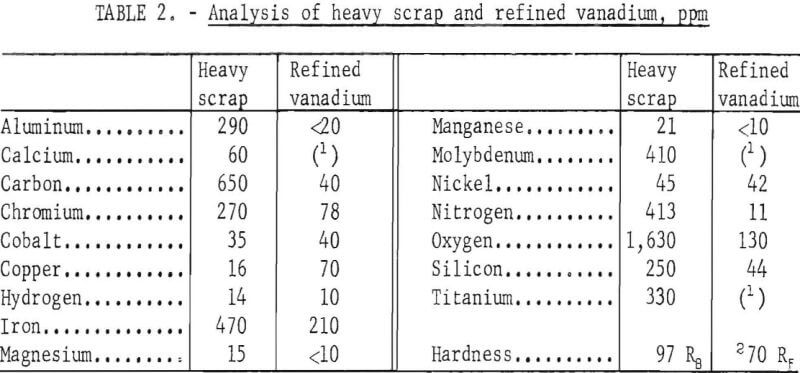
Table 3 shows the analysis of individual vanadium deposits refined at the beginning, middle, and end of series 1 and 2. The data reveal an interesting trend in which the impurity elements were transferred to the refined product. Chromium, iron, and nickel were gradually reduced in the middle of the series but increased near the end. Cobalt was not detected in the product until near the completion of refining. Copper content in the refined vanadium remained approximately at the same level as that in the scrap in series 1; its content in the product was higher in series 2. Both the iron and oxygen content in the vanadium increased substantially near the end of the series, coincident with a greater degree of cell feed depletion. The same trend existed in the refining products of series 3 and 4.
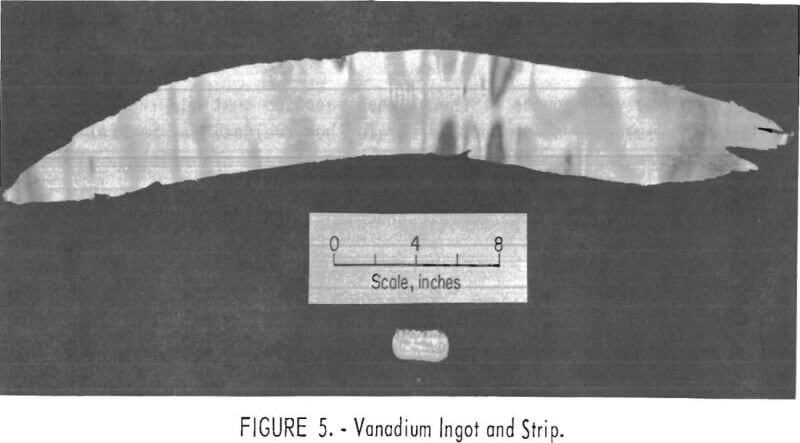
Approximately 18 pounds of the refined vanadium that had less than 400 ppm impurity elements was composited for further evaluation. Several 120-g ingots were prepared from this lot by inert atmosphere arc melting. The ductility of the vanadium was demonstrated by cold-rolling the ingots into 15- mil-thick strips without intermediate annealing. An ingot and cold-rolled strip are shown in figure 5.
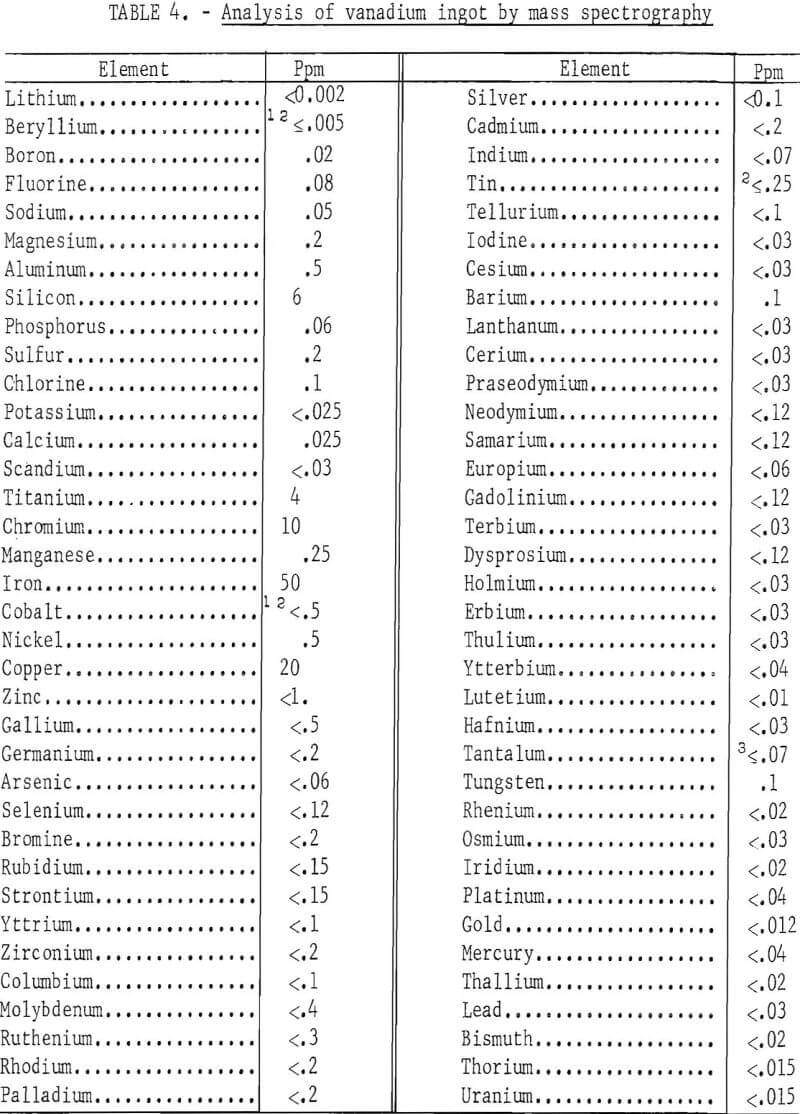
Another ingot from the same lot was analyzed by solid-state, spark source mass spectrography for substitutional impurities, except carbon, hydrogen, nitrogen, and oxygen. The maximum detectable metallic impurity content of the refined vanadium was 99 ppm. Table 4 gives the results of the analysis. This ingot had a hardness of 31 Rockwell F and contained 20 ppm carbon, 8 ppm hydrogen, 12 ppm nitrogen, and 104 ppm oxygen in addition to the metallic impurities.
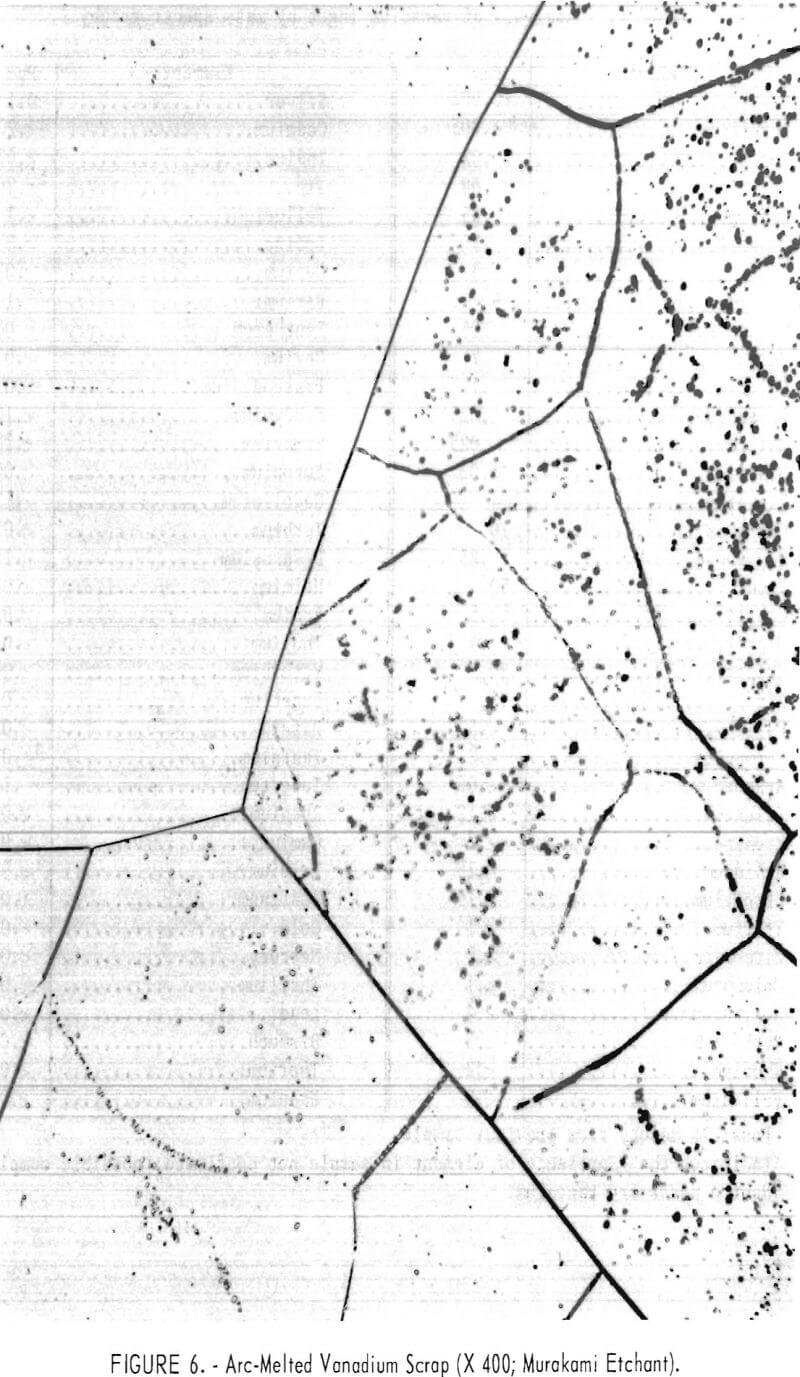
Figures 6 and 7 show typical photomicrographs of arc-melted vanadium scrap and arc-melted electrorefined vanadium product. Very little evidence of impurity inclusions is shown in the refined vanadium specimen.

Light Scrap
Refining
The light scrap was pressed into 1-inch-diameter buttons, about ½ inch thick, with a hydraulic pressure of approximately 400 lb/in² before loading into the anode basket. Table 5 shows the operating data for the light scrap. Eighty-three pounds of a new KCl-LiCl-VCl2 electrolyte were prepared which resulted in a molten-salt depth of approximately 12 inches. The cell operations were as effective as for the refining of heavy scrap. A little more than a pound of vanadium was produced in each test.

Refining produced 19.8 pounds of vanadium from 21.9 pounds of consumed scrap; 2.1 pounds of the scrap were lost as sludge, and 2.7 pounds of scrap were recovered. The electrolyte dragout with the anode basket was greater than in previous refining series, presumably owing to the shape of the light scrap which entrapped more electrolyte. The recovery of the refined vanadium was 90 percent with a cathode current efficiency of 94 percent. The average metal-to-electrolyte dragout ratio was 9.4 to 1.0 for the series.
Quality of Product
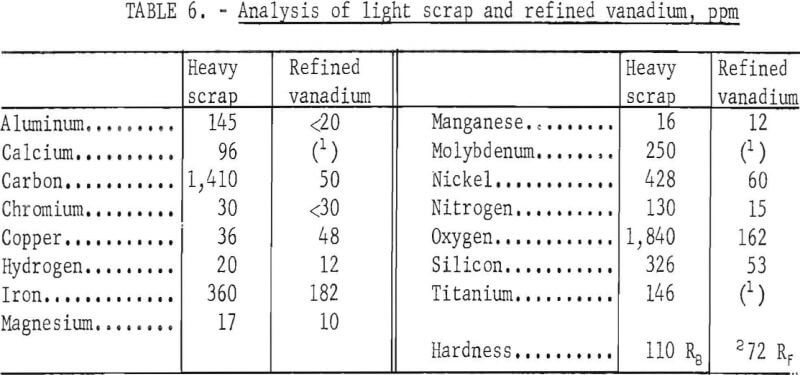
Table 6 presents the analysis of the light scrap and the refined vanadium. Impurity elements substantially reduced in the refining were aluminum, carbon, molybdenum, nitrogen, oxygen, silicon, and titanium. A slight reduction of chromium was made in the product. The copper content was not reduced. Nickel was reduced from 428 ppm to 60 ppm: The chief contaminants in the products were iron and oxygen and the concentrations of each element ranged over 100 ppm. The refined vanadium was ductile and had almost the same hardness value as the refined product produced from the heavy scrap. The product contained 654 ppm impurity elements compared with 5,250 ppm in the scrap.
Granular Scrap
Refining
The vanadium granules in the scrap consisted of plus 8- to plus 10-mesh vanadium. They were readily used as cell feed without further processing, as was necessary for the light scrap. Table 7 presents the operating data for this refining series. The electrolyte used was the same as was used in refining the light scrap. A KCl-LiCl eutectic salt mixture was added to the electrolyte before starting the refining to compensate for the electrolyte dragout from the previous series.

Refining produced 18.8 pounds of vanadium from 21.3 pounds of consumed granules; 2.5 pounds of the granules were lost as sludge, and 2.1 pounds of the scrap were recovered The electrolyte dragout with anode basket was the highest of all refining series. The vanadium recovery was 88 percent with a cathode current efficiency of 90 percent. The average metal-to-electrolyte dragout ratio was 7.8 to 1 for the series.
Quality of Product
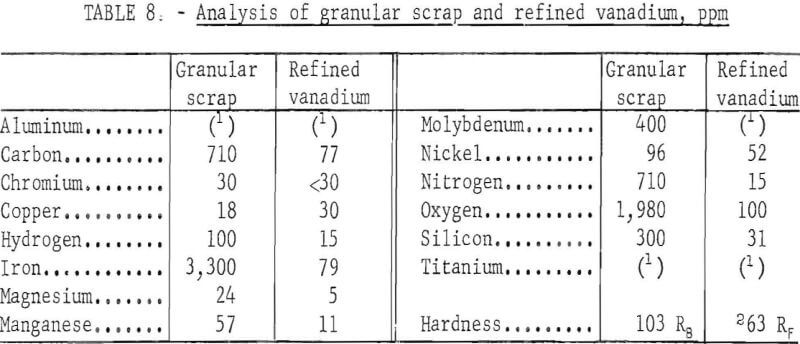
Analyses of the scrap granules and the refined product are presented in table 8. All impurity elements except copper, chromium, and nickel were reduced to a low level through the refinings. The chromium content was reduced to less than 30 ppm. The copper content of the refined vanadium was slightly higher than that of the feed, while nickel was reduced about 45 percent in the product. The chief contaminants in the product were iron and oxygen, but their contents were comparatively lower than those in the products produced from other series of refinings. The granules had a greater proportion of impurity elements, 7,725 ppm, than the heavy and light scrap, but refining produced the best quality of vanadium, with only 445 ppm impurity elements. The hardness of the vanadium was 63 Rockwell F, the lowest value attained in all series of refinings.
Discussion
Vanadium scrap was successfully converted into ductile vanadium with a 99.93- to 99.95-percent purity range in each refining series. The chief difference in the quality of the product refined from each type of scrap was the iron and oxygen content. While the vanadium refined from the heavy and light scrap contained in each instance a total of about 340 ppm of iron and oxygen, vanadium refined from the granular scrap had about 180 ppm. The product from the heavy scrap also contained a minor amount of chromium, copper, and nickel. The chromium was reduced to less than 30 ppm in the vanadium refined from light and granular scrap. The vanadium crystals refined from the scrap contained about 150 ppm of occluded alkali chlorides from the electrolyte, as shown by flame photometry analysis. Consolidation of the crystals into ingots by inert-atmosphere arc melting reduced these impurities to less than 10 ppm.
During each series of refining tests, at least four electrolyte samples were taken at definite intervals. These samples were taken between tests at a 2-inch depth in the molten electrolyte where the cathode was positioned. A nickel cup, which held about 30 g of electrolyte, was used for sampling. The electrolyte samples were analyzed for chromium, copper, iron, and nickel, as well as for the vanadium content and its average valence. The change of vanadium content in the electrolyte was minute, about 0.1 percent in a series of refining. The average valence of the vanadium in the electrolyte remained 2.0 to 2.1 during the refining series. The concentration of the four metallic impurities did not change appreciably; they totaled approximately 200 ppm. However, the lowest concentrations, about 125 ppm, were detected in the middle of the series, coinciding with the same low impurity level of the elements detected in the refined vanadium.
The major components of the anode sludges which were recovered from the anode basket at the conclusion of each series were similar in all series. They consisted of and VO0.2 with a minor component of V2C and were identified by X-ray analysis. Chemical analysis showed that the sludges contained up to 70 percent vanadium, 6 to 28 percent oxygen, up to 1 percent aluminum, carbon, iron, molybdenum, nitrogen, and silicon, and less than 0.1 percent chromium, copper, arid nickel. Some of the sludge which remained in the bottom of the cell was not recovered. For this reason the calculation of vanadium recovery from cell feed was based on the vanadium produced and the amount of scrap consumed in the refining. The difference between these two values was reported as sludge.
