Table of Contents
This series of investigations was exploratory in nature, as all variables that might influence alloy-element transfer were not examined. The test show, that, under the conditions described, titanium can be refined almost completely from molybdenum, tin, and zirconium; that refining from aluminum and chromium is only slightly less successful; and that refining from vanadium and especially manganese is relatively poor. The tests also have shown that a readjustment of operating variables or conditions is required to prevent electrowinning of titanium when it is refined from molybdenum and aluminum. Changes in operating conditions also are indicated if refining of titanium from aluminum, chromium, and vanadium is to be complete. The fact that manganese contaminated both the electrolyte and the sublimates that collect in the cold zones of the cell indicates that refining titanium from an alloy containing manganese will not be successful unless some means is found to render the manganese electrically and chemically inert. However, tests of the manganese alloy indicate that a manganese alloy of any desired composition could be deposited, if enough soluble manganese were present in the electrolyte.
The tests also show that removal at regular intervals of the scale formed on the anode during electrolysis would be desirable. The blanketing effect of the scale has three possible detrimental consequences: It can lower the cathode current efficiency, increase the tendency of alloying elements to transfer, and increase the danger of electrowinning of titanium from the electrolyte.
Of interest is the fact that the deposits recovered were much softer than the original material regardless of the alloy tested. The reduction in hardness is due largely to the elimination of interstitials and iron, which makes the electrorefining process desirable for purifying other alloys. Although the quantity of an alloying element might transfer unchanged, the purification of alloy scrap by electrorefining would be economically sound if a product were acquired whose ductility had been restored.
Some have been active in developing an electrorefining process to recover titanium of useful quality from offgrade Kroll sponge, mill and consumer scrap metal. Utilization of these products, which accumulated rapidly during the early growth of the industry, was necessary, both as a conservation measure and to reduce the price of titanium to the consumer. However, as the industry developed, operating techniques and blending practices improved. Consequently, less primary metal required salvaging, mill scrap became more amenable to remelting owing to availability of higher quality sponge, and the need for refining in these fields decreased. With production of higher quality primary metal, the number and complexity of titanium-base alloys increased, and disposal of mixed consumer scrap again became a major problem. Fused-salt electrorefining offers a possible solution to this problem.
In 1954, while developing the titanium electrorefining process, the Bureau attempted to electrorefine titanium from three complex commercial alloys. Certain anomalies were observed in the tests, particularly in the extent of transfer of elements common to two alloys. Even under the same operating conditions identical alloying constituents in separate alloys reacted differently. It appeared that the behavior of one element was influenced by the action of other elements in the alloy. Consequently, it was felt that an investigation to determine the transfer behavior of an individual element alloyed with titanium would provide basic information and that such tests might serve as a foundation upon which to predict the transfer characteristics of each element in complex titanium-base alloys. The identifiable characteristics of each binary alloy also might indicate electrorefining requirements for obtaining a product of a desired composition.
Binary-Alloy Preparation
For this investigation, the Federal Bureau of Mines Northwest Electrodevelopment Experiment Station, Albany, Oreg., prepared 7 binary titanium alloys with a nominal composition of 95 percent titanium and 5 percent alloying element. The titanium was electrorefined metal with an average Brinell hardness number of 90. The alloying elements – aluminum, chromium, manganese, molybdenum, tin, vanadium, and zirconium – were as pure as were commercially available.
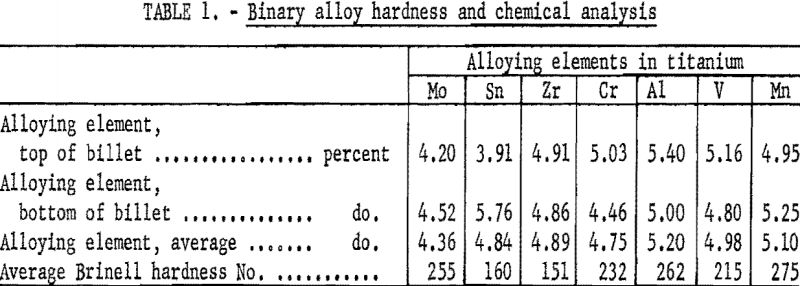
Before the as-received billets were prepared for anodes, each billet was turned smooth in a lathe, and the ends were cropped to remove surface imperfections and any metal that might have been contaminated by the water-cooled mold or tungsten electrode. The ends of each billet were then sampled individually by taking light lathe turnings from across both faces. The chips and turnings from each face were mixed thoroughly and separately and analyzed in duplicate. The analyses in table 1 are averages of the duplicate analyses. The hardness values shown for each end are the average of two impressions. The cylindrical alloy ingots cast by the Albany Station were quartered lengthwise to form 4 equal anodes 6 inches long and having a pie-shaped cross section.
Electrorefining Procedure
The procedure called test method 1 in the tables was designed to obtain data on the effect of cathode current density on distribution of the alloying component. (The complete cycle of producing a cathode deposit and removing it from the cell constitutes a test for the purpose of this paper.) A series of deposits was produced, in which the initial cathode current densities were increased from deposit to deposit with a predetermined theoretical quantity of the anode to be transferred in each test. The anode was removed from the cell between individual tests, cleaned, and weighed, and samples of the anode surface residue were analyzed to determine the distribution of the alloying component. In addition, periodic analyses of the electrolyte and sublimate were made to determine the concentration of the alloying element. This technique also permitted maintenance of a fresh anode surface for dissolution and inspection of the type of scale or sludge that developed on the anode during electrolysis.
Specifically, in the first method, 5 tests were made on each alloy using initial cathode current densities of 130, 200, 200, 300, and 500 amperes per square foot. The desired quantity of the original anode weight to be transferred in all the tests was 5 percent, except in the second 200-ampere test, when the desired quantity was 8 percent. The test procedures for the first two elements studied in this investigation, aluminum and chromium, did not adhere strictly to the pattern; however, the transfer characteristics were such that additional tests were not required.
Test method 2 was designed to ascertain the effect of the anode scale on the extent of transfer of titanium and of the alloying component. A series of five cathode deposits was produced at a constant initial cathode current density of 500 amperes per square foot, without removing and cleaning the anode between electrolyses. In each test it was desired to transfer a calculated 10 percent of the initial weight of the anode, based upon the ampere-hours of electrolysis applied at an expected current efficiency of 75 percent.
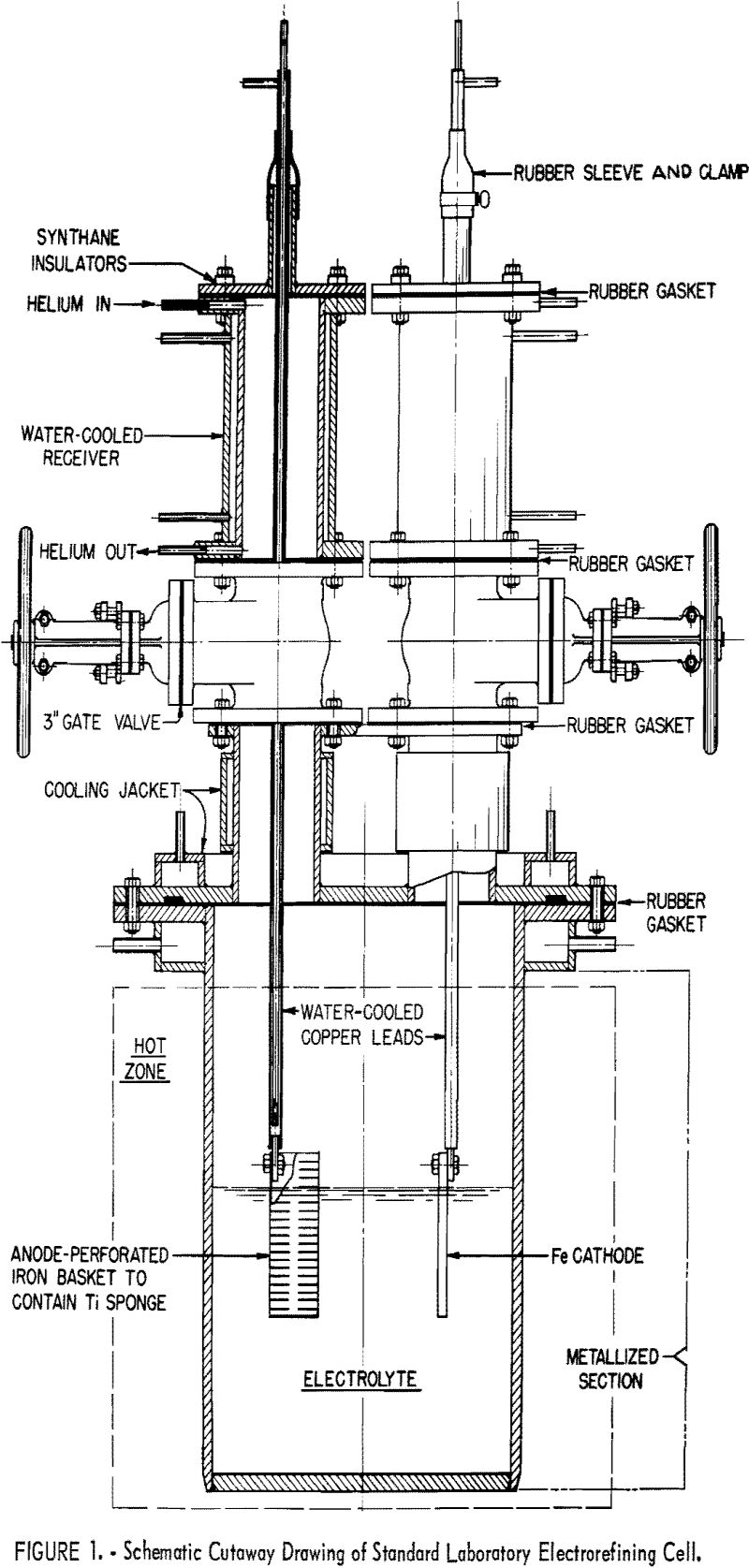
The investigations, represented by both testing methods, were conducted in a 12-inch-diameter laboratory cell (see fig. 1), containing an electrolyte composed of sodium chloride and 12-½ percent by weight of titanium dichloride. The temperature of operation for all tests was maintained at 850° C. ± 5°. The deposits recovered in both methods of testing were freed of electrolyte by leaching in a solution of water and hydrochloric acid (5 percent by volume), then washed with demineralized water until free of chloride ions, as indicated by the silver nitrate test, and dried. The dried titanium metal was separated into three fractions – plus 28; minus 28 to plus 48; and minus 48-mesh – by screening. Where material was sufficient, these fractions were sampled for chemical analysis and hardness evaluation.
Discussion
With the quantity of alloying element transferred to the cathode under similar conditions as a criterion, it was possible to divide the 7 elements into 3 relatively distinct groups. The quantity of molybdenum, tin, or zirconium transferred was negligible , as the maximum percentage of the alloying element found in a deposit was 0.09. The second group comprised chromium and aluminum. The extent to which these elements transferred approximated that of the first group under the conditions of test method 1; however, under the conditions of test method 2 the quantity of the alloying element transferred increased materially over that of elements of the first group. The maximum percentage of alloying element found in a deposit was 0.54 for
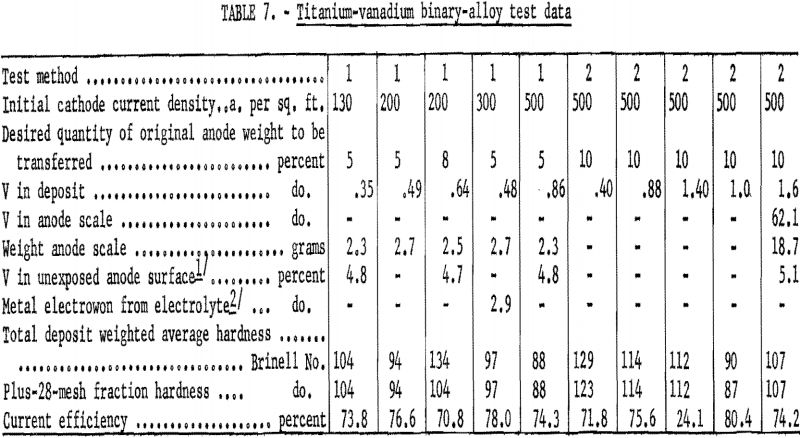
the aluminum alloy and 0.75 for the chromium alloy. Manganese and vanadium, which constituted the third group, transferred to the greatest extent. The highest percentage of vanadium found in a deposit was 1.64 and of manganese, 2.71.
During tests of some alloys the quantity of metal recovered in the deposits exceeded that removed from the anode. In these instances the metal could only have been electrowon from the subvalent chlorides in the electrolyte.
The incidence of electrowinning may be a function of anode current density, of quantity of soluble titanium in the electrolyte, or of transfer characteristics peculiar to the alloying element under investigation. This phase of the alloy-refining study will be the subject of a further investigation.
Elements in the First Group
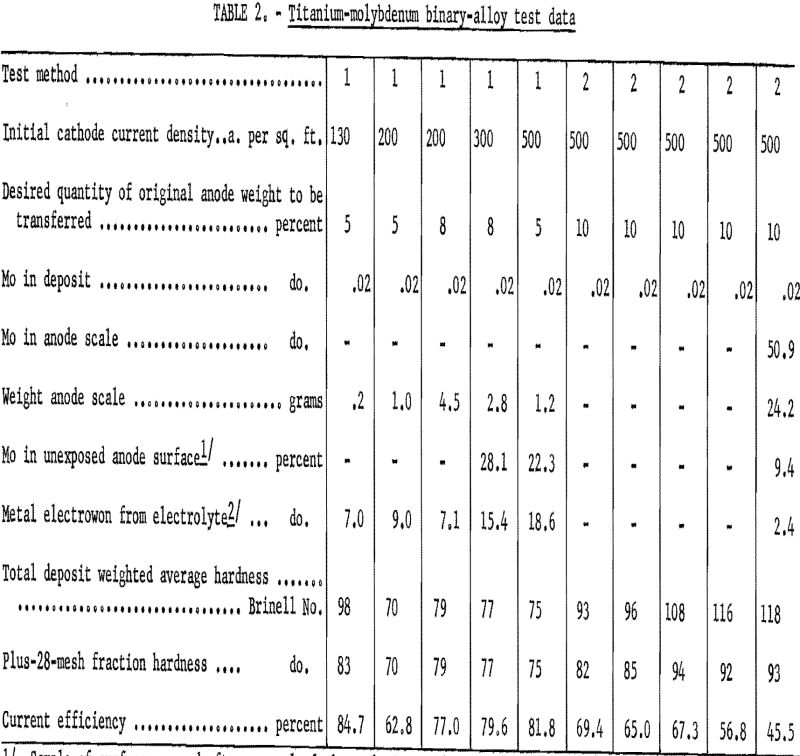
Elements comprising this group are molybdenum, tin, and zirconium. Table 2 gives test data for the molybdenum-alloy studies. Titanium was refined from the molybdenum-alloy billet, regardless of operating conditions, and no cathode deposit contained over 0.02 percent molybdenum. Cathode current efficiencies in test method 1 exceeded the anticipated 75 percent, with one exception. However, in test method 2, current efficiencies, with one exception, dropped with each succeeding test. This condition probably was caused by the manner in which molybdenum functioned at the anode. The scale that remained on the anode after each test of the test method 2 series was thin, hard, and difficult to remove. It was necessary to machine the anode on a lathe to remove the scale. The integral surface immediately under the scale was greatly enriched in molybdenum. One sample of metal turned from this surface contained 28 percent molybdenum, indicating that the molybdenum was concentrating by diffusion into the unexposed anode, making it essentially seminoble and causing electrowinning as well as electrorefining of the titanium. The decreasing current efficiencies evident in the test method 2 series are due to reoxidation of deposited metal caused by electrowinning of titanium from TiCl2 in the electrolyte.
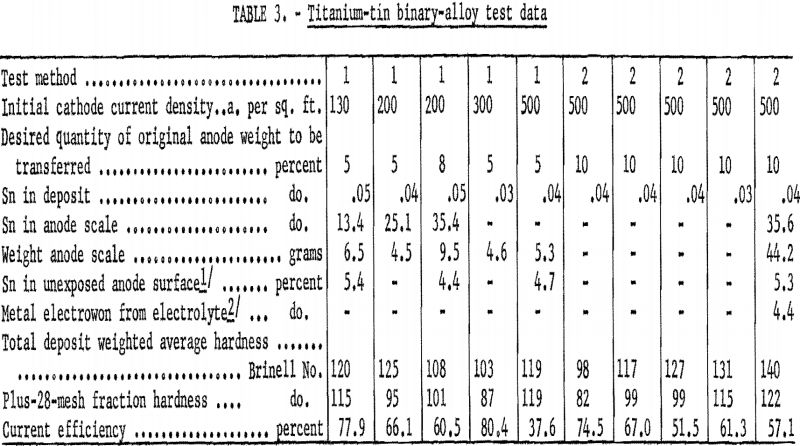
Table 3 gives refining data on tin, the second alloying element of the first group. Titanium was refined from the tin alloy almost as well as from the molybdenum alloy. The maximum tin found in a deposit refined from the tin alloy was 0.05 percent, compared with 0.02 percent for molybdenum. The scale that formed on the tin-alloy anode was similar to that formed on the molybdenum anode in hardness and difficulty of removal for analysis. The quantity of adhering anode scale was approximately twice that found on the molybdenum anode, but the tin had not diffused into the anode as had the molybdenum. One analysis of the scale showed a tin content of 35.6 percent. Electrowinning from the electrolyte did not occur in test method 1 but did occur in test method 2, when the anode surface became blanketed with scale.
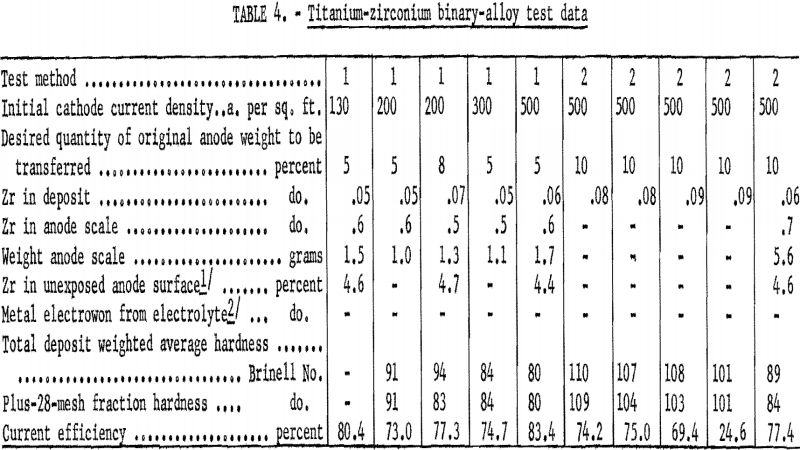
Table 4 presents data from both methods of testing zirconium, the third alloying element in the first group. The highest percentage of zirconium found in a deposit was 0.09.
The scaling properties of the zirconium-alloy anode differed from those of either of the first two alloys. Analysis of the scale showed it to have the same nominal composition as the original alloy, and there was no indication of concentration by diffusion of zirconium deeper into the anode during electrolysis. Indications were that the zirconium left exposed as the titanium was dissolved from the anode dropped from the anode into the bottom of the cell. This phenomenon may be one explanation of why no electrowinning occurred in any of the zirconium-alloy tests.
Elements in the Second Group
Chromium and aluminum were placed in the second group because the extent of their transfer, especially in test method 2 was intermediate between that of the first and third group.
Table 5 presents pertinent data taken while testing chromium. The quantity of chromium in deposits formed in test method 1 never exceeded 0.15 percent. Deposits formed in test method 2 contained appreciably more chromium, the quantity increasing progressively to a maximum of 0.75 percent as the scale blanketed the anode. Cathode current efficiencies decreased progressively under test method 2, again owing to the blanketing effect of the anode scale. However, the scale did not cause electrowinning of titanium. Chromium concentrated in the anode scale, which was dense and hard, but it did not diffuse into the anode or contaminate the electrolyte.
Table 6 gives the results of study of the transfer behavior of aluminum. Unlike chromium, the other element in this group, the extent of the aluminum transfer in test method 1 increased fourfold when transfer of the original anode metal increased from 5 to 8 percent. The aluminum alloy also differed from the chromium alloy in that electrowinning occurred in every test, regardless of operating conditions. Cathode current efficiencies were lower than anticipated in most of the tests, especially in the test method 2 series. The scale formed at the anode differed from all others in the binary examination, being in two layers. The soft outer layer of scale remaining after the test method 2 series was easily scraped off for analysis and contained 11.7 percent aluminum, whereas the dense, hard, 1/8-inch-thick inner layer contained 16.5 percent aluminum. The inner layer had the surface appearance of solid metal but was gray instead of silvery and could only be removed by machining the anode on a lathe. The difference in character of the scale formed on the chromium and aluminum alloys probably accounts for the fact that, although more chromium transferred, electrowinning was greater and current efficiencies were poorer with the aluminum alloy.
Elements in the Third Group
Refining of titanium from the third group – vanadium and manganese – was poor compared with the success achieved with the first and second groups. Table 7 gives data taken during testing of the vanadium alloy. Cathode current efficiencies approximated the anticipated 75 percent, except in one test, in which most of the deposited metal was scraped off the cathode as it was lifted into the receiver to cool. All the metal recovered during the tests was electrorefined with one exception. There was no apparent reason for the deviation of this one test, which indicated that electrowinning had taken place. In the test method 1 series, vanadium concentration in the deposits increased progressively as the cathode current density was increased. The concentration also increased with each test in the test method 2 series, as the anode became blanketed with scale. Vanadium concentrated in the small quantity of dense, hard scale that remained after electrolysis but did not diffuse into the anode or contaminate the electrolyte, as the scale sloughed off the anode.
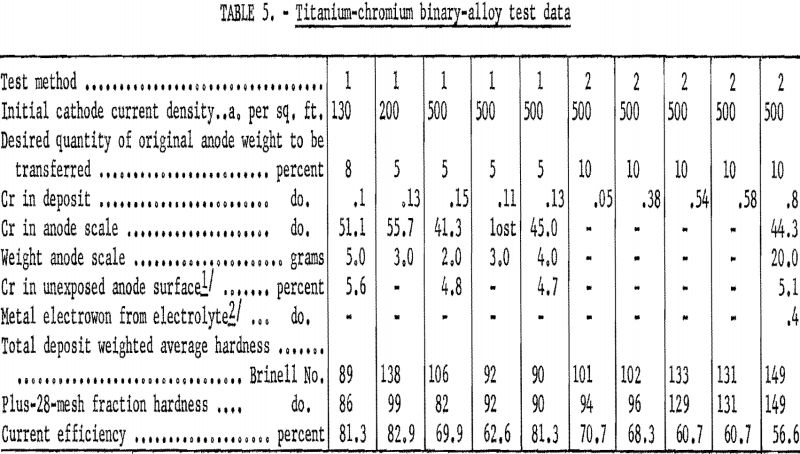
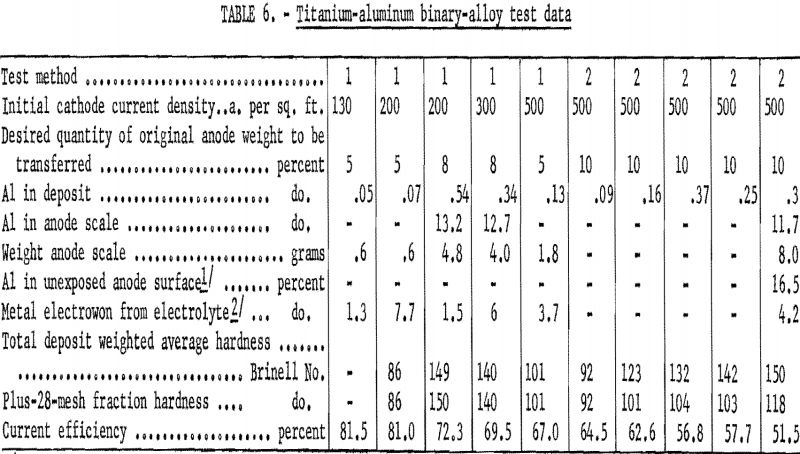
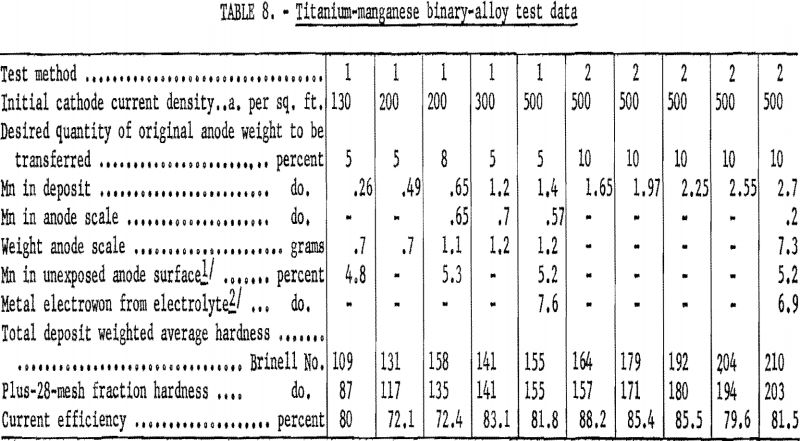
Table 8 gives the data on the transfer characteristics of manganese, the alloying element that transferred to the greatest extent. Cathode current efficiencies of the manganese-alloy tests, like those with vanadium, were as anticipated. Electrowinning was not a factor in test method 1 except at the highest cathode current density, and only occurred to a slight extent in test method 2. Manganese did not concentrate in the slight quantity of scale formed or diffuse into the anode, but it did contaminate the electrolyte. The greatest quantity of manganese recovered from the anode scale was 0.72 percent – considerably less than was present originally in the alloy anode. At the beginning of the manganese-alloy study, the electrolyte did not contain a detectable quality of manganese. At the conclusion of the study the bath contained 0.09 percent manganese, and the sublimate that collected in cold sections of the cell contained 0.05 percent manganese.