Table of Contents
The Federal Bureau of Mines refined chromium from an aqueous electrolytic process in molten sodium chloride-chromium chloride electrolytes. Oxygen and nitrogen contents were reduced by a factor of 10 to 40, but most other impurities were little changed. The product usually contained a little more iron than the feed. Since iron was entering the bath from the iron pot and the graphite liner at a nearly constant rate, contamination of the chromium deposits by it was more serious at low currents and deposition rates. A few runs in an iron cell with a nickel liner yielded chromium containing one half as much iron as the feed, but up to 1.5 pct. nickel. In all cases the anode feed lay in the bottom of the container, which itself was used as the anode connection.
With a 12-in. cell, variations in applied voltage from 0.05 to 1.5, in current from less than 10 to over 100 a., and in initial cathode current densities from less than 100 to over 2,000 a./sq.ft. had little or no effect upon the analysis of the metal produced, except for iron content. Changes in bath analysis from 3 to 11 pct. chromium were also without effect. These changes did bring about large differences in the physical forms of the crystals obtained. The thick heavy crystals produced at low voltage and current contained voids which made them difficult to leach free of chlorides. Easiest to leach were the thin leaf-like crystals obtained at around 1 v.
The crystalline chromium obtained was somewhat ductile but did not remain so after being melted into buttons tor hardness testing. The average hardness of the feeds was 75, and of the products, 72 Rockwell B. This small difference between materials with large differences in oxygen and nitrogen contents, plus some lack in reproducibility of results, leads to the conclusion that the present method of hardness testing does not affort an index to the oxygen and nitrogen content of chromium metal.
Current efficiencies of 90 pct. were normal, but excessively large deposits, or deposits of extremely large crystals, were usually in part lost to the bath, with the result that current efficiencies apparently as low as 55 pct. were at times obtained.
The average metal-to-salts weight ratio of deposits removed from the cell was 2.4 to 1.
Introduction
In the past few years industrial and governmental research organizations have undertaken many studies for the development of methods for producing many metals in the purest state possible. This has come about with the recognition that metals that are highly pure often have properties that are different- sometimes predictably different and predictably more useful— from those they have in the ordinary state. Work on the purification of metals has been intensified with the advent of the nuclear and space age.
Uses of pure metals and their alloys are not restricted to structural needs. There have been such applications as the use of irradiated chromium for tissue implants in the fight against cancer. There are, moreover, several direct applications for high-purity chromium in fields that are as yet of a classified nature.
As part of its overall program for the better utilization of our national resources, the Bureau of Mines has studied the application of the technique of fused-salt electrorefining to chromium. This technique has proved successful in the purification of many metals, such as titanium, zirconium, hafnium, vanadium, and beryllium, and offers opportunity for the production of high-purity metals in quantity.
Equipment
Experimental work began In 3- and 4-in.-diameter cells, and the bulk of the tests were conducted in a 12-in. diameter cell.
Figure 1 is a schematic diagram of the electrolytic cells used. With the exception of the use of graphite liners, or in one instance of a nickel liner
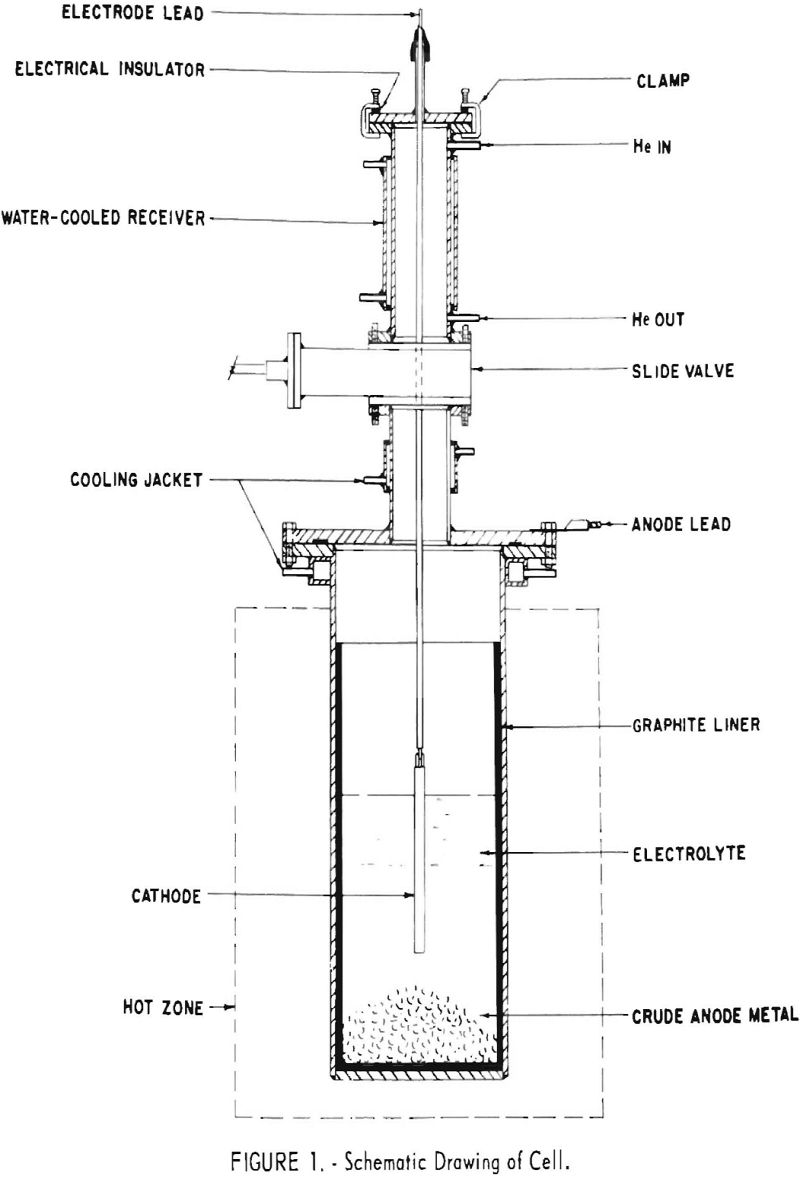
to prevent contamination of the bath with iron, these cells were identical to those used for the salt-bath refining of titanium. A receiving chamber and valve permitted the introduction and removal of the cathode and the introduction of salt or anode feed without disturbing the inert (helium) atmosphere of the cell proper. External electric heat was used to maintain the electrolyte at a little over 800° C.
In all cells, flakes of chromium metal about one eighth of an in. thick and up to about 2-½ in. square were charged to the bottom of the cell for the anode. The cathodes for the smaller cells were ¼-in.-diameter molybdenum rod. Those for the 12-in. cell were in nearly all cases ¾-in.-diameter iron rods, commonly immersed 4 or 6 in. into the bath. Experience soon showed how much current these combinations of cathode and effective anode areas would support without leading to poor cell operation and a degraded product.
Electrolytes
Molten sodium chloride solutions of chromium dichloride were used as electrolytes for these studies. In all cells but the first the chromium chloride was added by the reaction of hydrogen chloride or chlorine, plus a little hydrogen chloride, with chromium metal in a suitable reactor, usually of graphite, immersed in the sodium chloride bath. The gases which left the cell passed through a bubbler containing oil and then through one containing a measured amount of standard potassium hydroxide solution and some methyl red indicator as a rough measure of unreacted chlorine or hydrogen chloride. The volume of chlorine and/or hydrogen chloride coming off the cell was kept down to 1 or 2 l./hr. Chemical analyses and current efficiencies showed that most of the soluble chromium in the bath was in the divalent state.
Experimental Procedures
A 3-in.-diameter cell of about 2-½-in. Inside diameter was used In the first experiments with an electrolyte composed of sodium chloride and ordinary crystalline chromium chloride. Water was removed from the mixture at low temperature under vacuum, but unfortunately some hydrolysis had occurred. The formation of oxychlorides caused the cell to produce a poor grade of chromium. Only a few of the fine cyrstals were bright, and the bath soon was abandoned.
The next cell was 4 in. in diameter with an inside diameter of 3-3/8 in. The electrolyte was sodium chloride and anhydrous chromium chloride, produced in the 3-in. cell.
The cell produced bright needles, dendrites, and plates of chromium from the start, at voltages of 0.1 to 0.6 and currents up to 11 a., on 1 to 2 sq. in. of cathode. The bath contained about 4 pet. chromium. An operating difficulty with this small cell was that the amount of salt removed with deposits, though not excessive, necessitated frequent additions of salt and chromium chloride to maintain the bath level and made control of bath composition difficult.
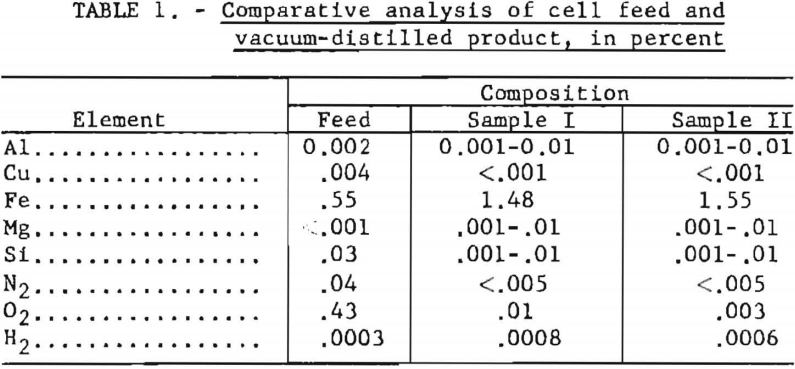
Two samples of the product were vacuum-distilled to remove salts and were sent to the Albany Metallurgy Research Center of the Bureau of Mines for evaluation. Analyses of these and of a representative sample of feed material are presented in table 1. Results, except for iron, oxygen, nitrogen, and hydrogen, are spectrographic.
This cell was shut down after proving the practicability of electrorefining chromium in a fused-salt bath. Cells as small as this are not well suited for quantitative studies.
The next cell was 12 in. in diameter, with a working diameter of 10 in. inside the graphite liner. The access valve and the receiver above it were 8 in. in diameter. All of this and more was needed, for some of the deposits obtained were so bulky that they did not wholly clear.
The initial electrolyte weighed 70 lb. and contained 6.3 pct. soluble chromium, and electrolytes containing from 3.2 to 11 pct. chromium were studied. The quantity of anode metal was maintained between 10 and 20 lb. of -½-in. chrome plate produced by aqueous electrolysis process. Applied cell voltages ranged from less than 0.1 to 2.6, and cell currents, controlled by applied voltage and by cathode immersion, ranged from a few a. up to 150 a. Current densities at the cathode, usually ¾-in.-diameter bar stock, ranged from less than 100 to over 3,000 a./sq.ft. There was also some manipulation of bath temperatures, from 800° C. to 860° C.
These changes did not make any great differences in the purity except for iron content, or in the brightness of the product, provided that the useful current capacity of chis cell and bath, about 100 a., was not exceeded. A run at the excessive constant current of 150 a. and the comparatively moderate cathode current density of 1,400 a./sq. ft., with a voltage that varied from 1.9 to 1.35, yielded metal that was dirty grey in appearance. This metal was high in impurities, especially oxygen, because of excessive anode current density and voltage drop. Chemical analyses, except for iron content, were not made.
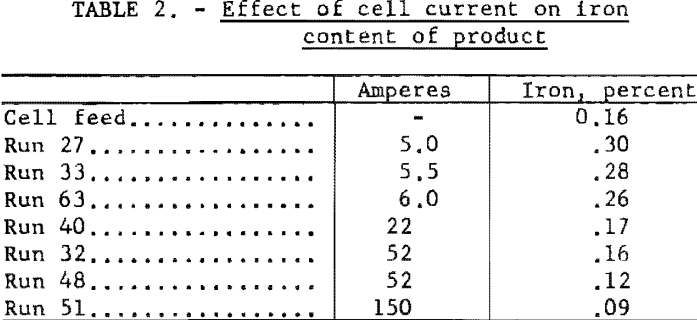
The chromium metal produced at low currents and deposition rates contained a greater percentage of iron than that produced at high deposition rates, apparently because iron was migrating into the bath and the deposited metal at a slow but more or less constant rate regardless of the current through the cell. Table 2 illustrates this. In some later runs, not tabulated in this report, the effect was obscured by the use of a feed that was much higher in iron.
There is one other possible source of iron contamination. This is from the materials of construction, mainly the iron cell and/or the graphite cell liners.
The possibility that iron contamination was resulting from the iron cell through a vapor-phase phenomenon was considered, as the liners did not show any evidence of the electrolyte having leached through the crucibles. To determine the potential of this vapor-phase possibility, the following theoretical calculations were made, assuming a cell temperature of 827° C. (or 1,100° K.) . The values used in these calculations for the constituents involved were:
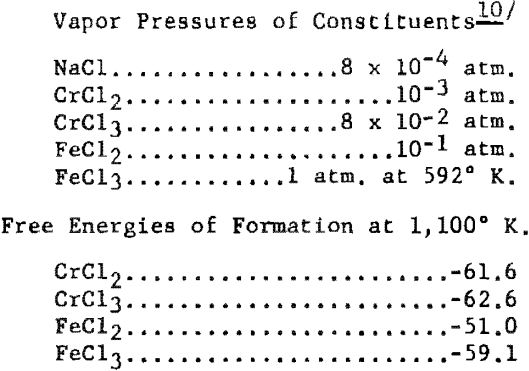
It was assumed that some CrCl3 could be made at the anode. Also, if the mole ratio of soluble chromium was such that the average effective valence of the chromium was 2.1, the mole fractions of the components would be 0.94 for NaCl, 0.056 for CrCl2, and 0.0063 for CrCl2. The activity coefficients for these materials in fused salts are about 1.1 x 10-² for CrCl2. 1.6 for CrCl3, and about 10-² for FeCl2. Thus the activities of the chromium compounds would be 6.16 x 10 -4 for CrCl2 and 1.0 x 10-² for CrCl3. From this, the calculated vapor pressures of each compound above the fused salt would be 0.6 mm. for CrCl3 and 4.7 x 10 -4 mm. for CrCl2. It would appear that 0.6 mm. pressure of CrCl3 would be sufficient for a considerable amount of this substance to diffuse to a surface containing iron.
The possible reactions that could then take place are:
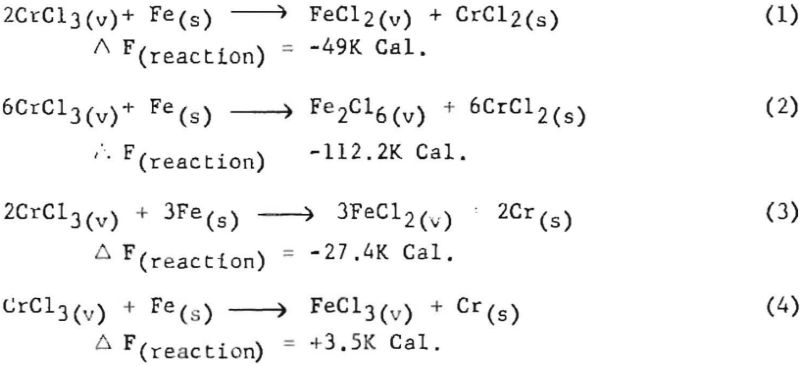
In reactions 1, 2, and 3 above, Ka (equilibrium constant in terms of activity) calculation shows that the CrCl3 has enough activity to give the products an activity value of greater than unity. This means that if equilibrium were reached FeCl2 or Fe2Cl6 would have partial pressure in the vapor state corresponding to the conditions of the temperature of condensation or that FeCl2 or Fe2Cl6 would have a relatively high concentration in the gaseous state to return to the fused-salt bath.
The limit of the reaction would be the rate of diffusion of CrCl3 from the fused-salt bath to an iron-containing surface. After the iron chlorides are formed and have diffused back to and entered the fused salt, iron would be readily deposited at the chromium cathode.
The amount of iron in the salt and available for deposition, since it is a kinetic problem, would depend on the duration of the runs, down time, cold surfaces available for iron chloride deposition, etc.
In an attempt to avoid some of the difficulties, including iron contamination associated with graphite liners, a dozen runs were made in a 4-in. cell with a nickel liner and a bath containing 3.8 pct. chromium. With an anode feed containing 0.55 pct, Iron, the products contained an average of 0.24 pct, iron. This degree of beneficiation could perhaps be bettered by manipulation of voltage, current, and bath strength. Since the deposits also contained up to 1.5 pct. nickel, the general use of that metal in chromium-refining cells without separate anodes is ruled out.
Average current efficiencies of 90 pct. were normal and readily attainable with attention to cell voltage, current, and total ampere hours per run. The excessive bulk of deposits containing over 200 g. of metal and the heavy and loosely hung character of the crystals obtained at low voltage and current caused losses to the bath, which resulted in apparent current efficiencies that were erratic and at times as low as 55 pct. Calculated efficiencies are based upon the assumption that all of the soluble chromium chloride in the bath is of valence 2.
A factor of concern in the operation of any salt bath cell is dragout, or the amount of salts removed with metal deposits and subsequently lost. The average metal-to-salt ratio for the 12-in. cell was 2.4 to 1. An operating temperature too close to the freezing point of the bath will of course increase the loss of salts. The average electrolyte temperature used was 20° higher than the melting point of the electrolyte, or about 820° C.
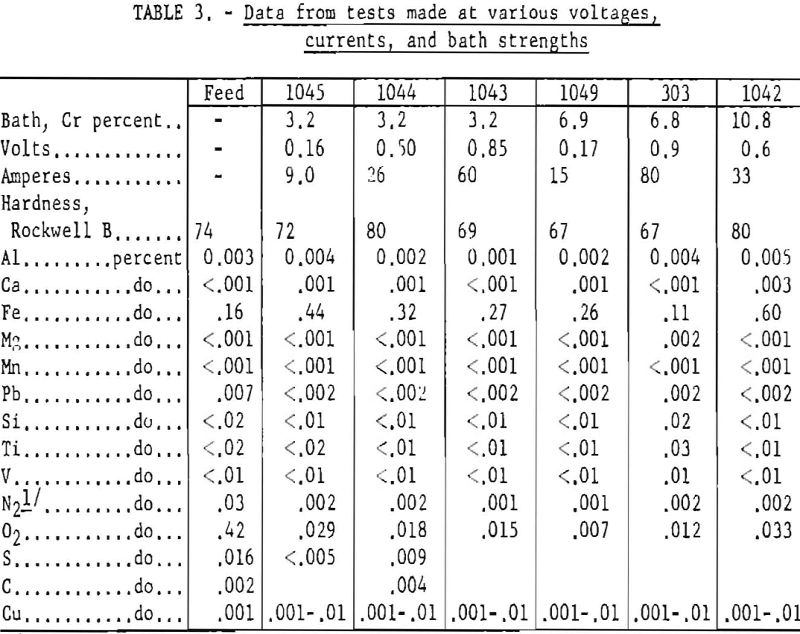
Table 3 shows operating conditions and chemical analyses for runs made at various voltages, currents, and bath strengths. Analytical results are spectrographic except for iron, nitrogen, oxygen, sulfur, and carbon. These runs were made with a single batch of feed material. Another batch, which contained 0.55 pet, iron but was otherwise similar, yielded products containing 0.6 to 0.8 pct, iron. This was the teed that in a nickel-lined cell yielded a product containing 0.24 pct. iron.
The iron content given for the product of the bath containing 10.8 pct, soluble chromium is an average. Runs from this bath showed iron contents which varied erratically from 0.36 to 0.81 pct. without relation to cell current. These high results were probably caused by a higher chromium chloride vapor pressure above the bath and a more rapid transfer of iron from the walls of the cell.
Some sulfur, lead, and probably silicon have been removed. It is reasonable to suppose that beneficiation with respect to these and some other impurities would have been more conspicuous with a less pure feed material. In particular, metals that are much more reactive than chromium would be less likely to deposit and metals that are much less reactive probably would not dissolve from the anode.
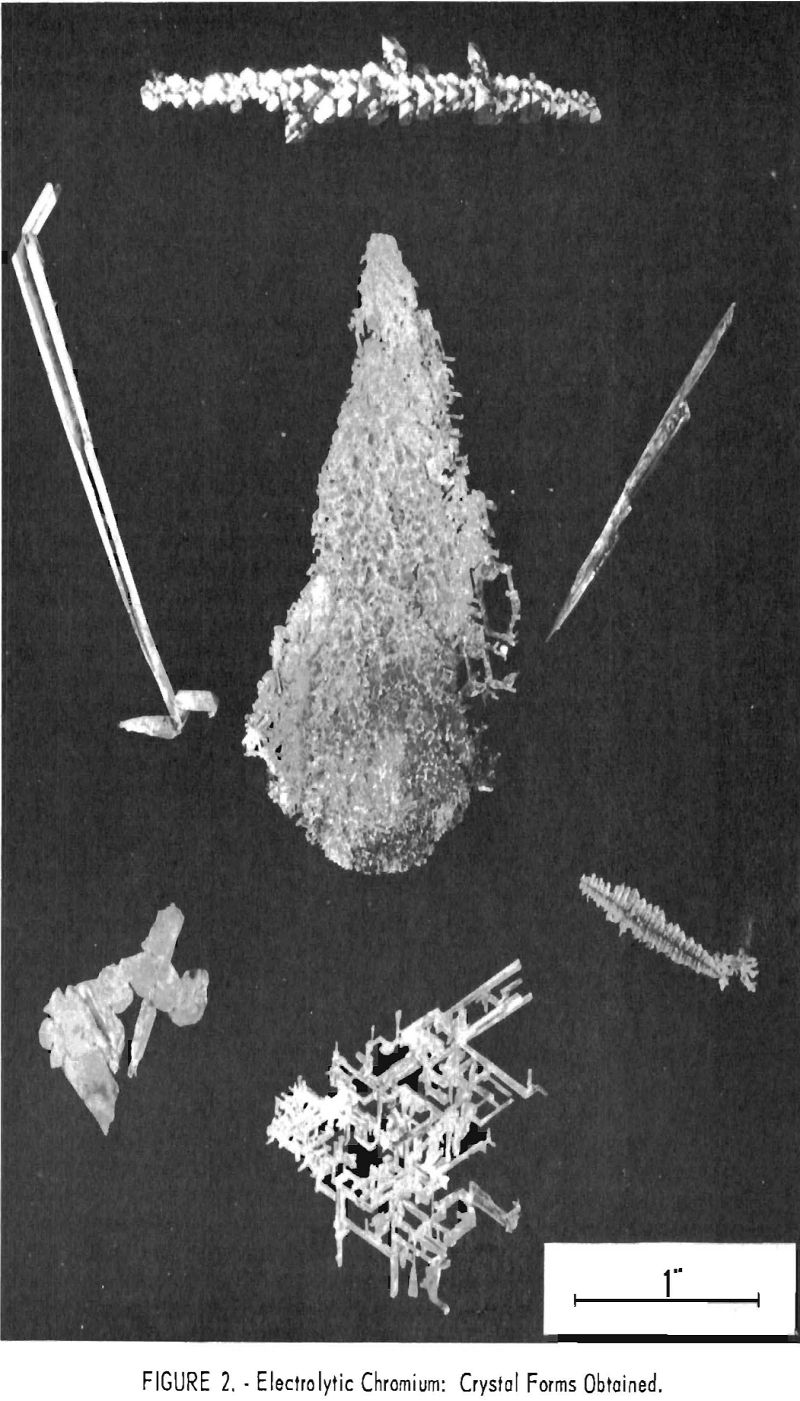
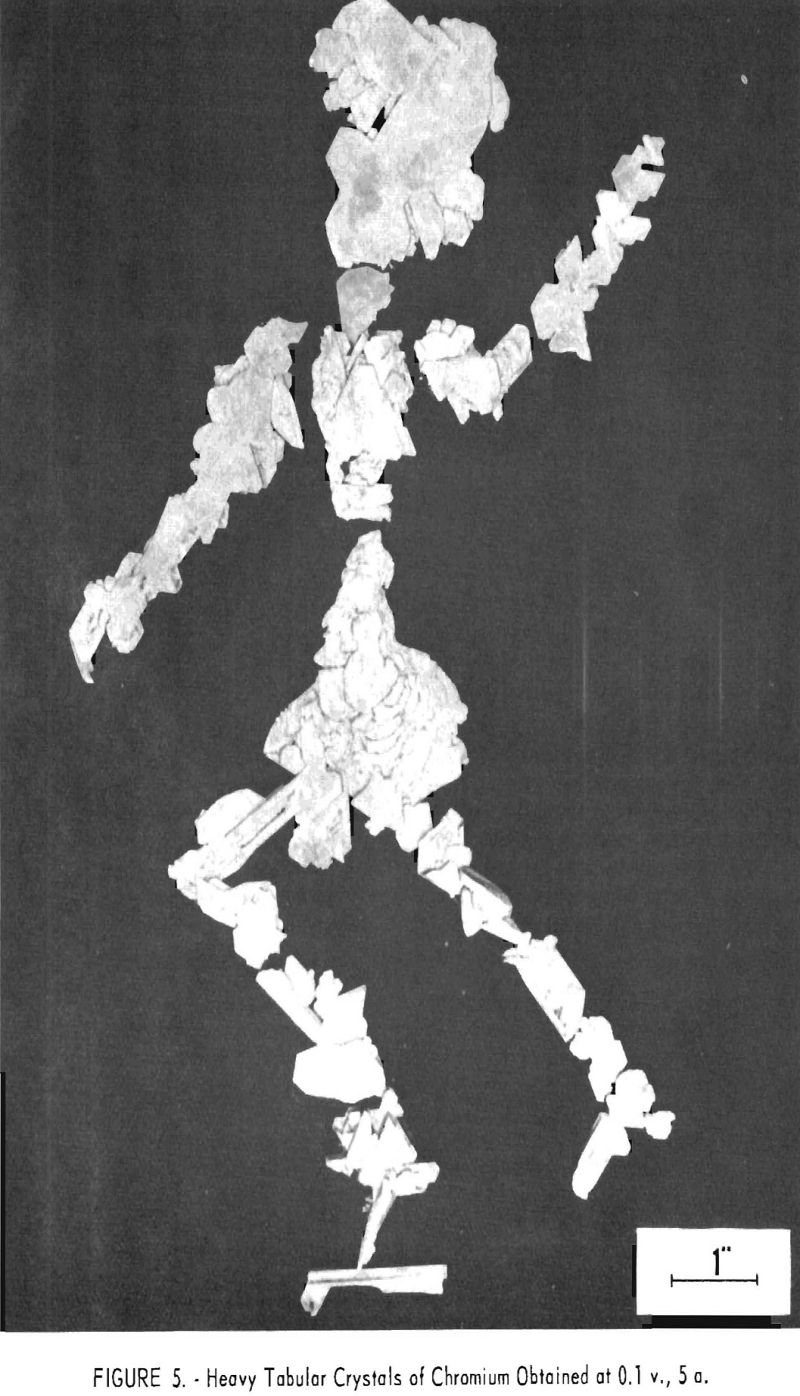
Manipulations of voltage, current, and bath strength made but little systematic difference in the analysis of the metal produced with respect to either metallic or nonmetallic impurities but did make extraordinary and in general reproducible differences in the crystal forms obtained. These varied through light and heavy needles, or reticular masses of needles, to dendrites, pyramids, thin lamellar leaves, and heavy plates, as shown in figure 2. Figures 3 and 4 show typical cathode deposits obtained at two different operating conditions. The heavy needles, pyramids, and plates obtained at low voltage and current were undesirable because they contained voids which made them difficult to leach free of salts, and because of their lower production rate. Figure 5 is typical of the heavy plates. The most desirable forms on both counts were the lamellar leaves obtained at rather high voltages and currents, for example 1.0 v. and approaching 100 a. When beaten under water with a high¬speed stirrer, these easily separated into thin, readily leached flakes. All leaching was done first with water containing a few percent hydrochloric acid and then with distilled water.
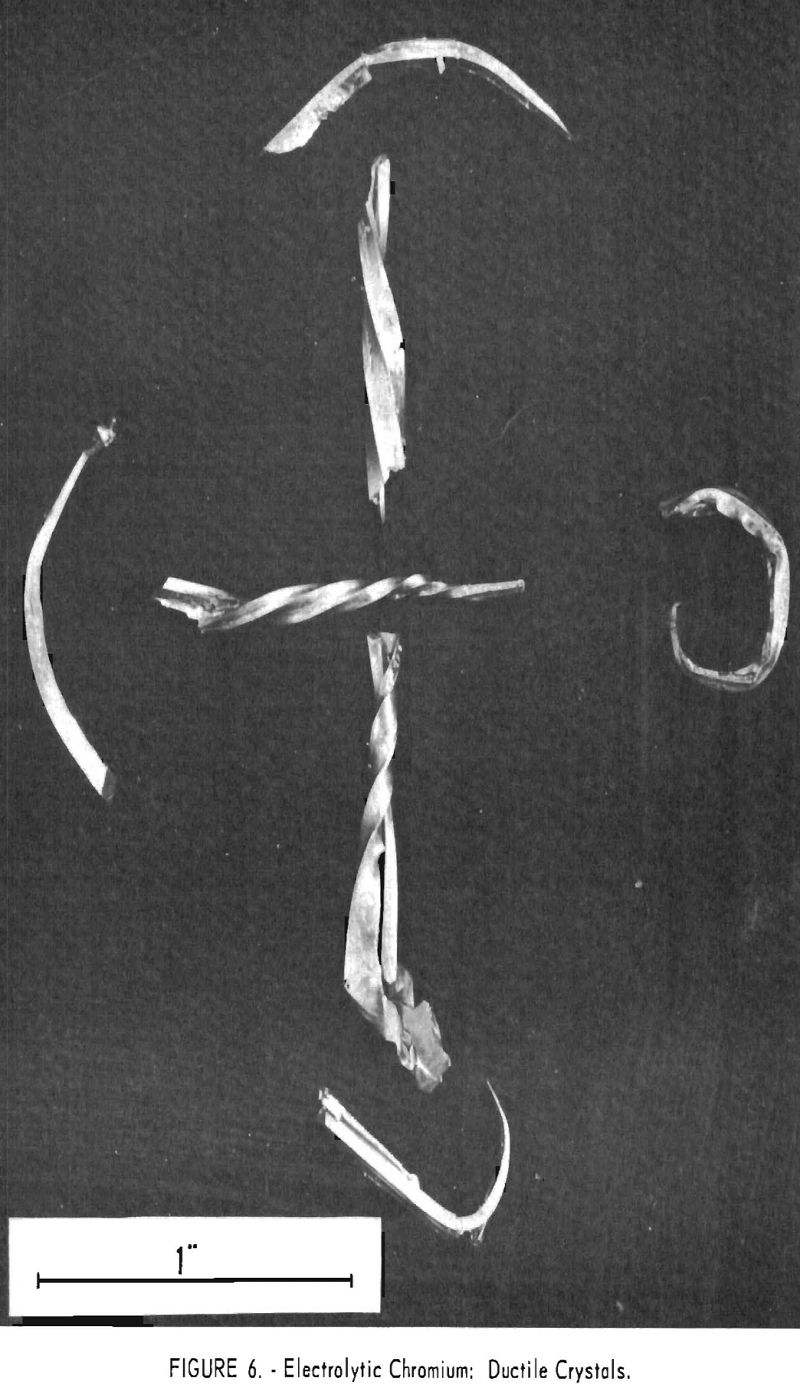
The average hardness of the feeds was 75 Rockwell B and of the products was 72, or substantially the same, despite the fact that the feeds contained a total of 5 to 50 times as much oxygen and nitrogen. Duplicate samples showed as much as 8 points difference in hardness. Crystals of chromium were somewhat ductile when produced, as figure 6 shows, but after they were melted into buttons for hardness testing their ductility vanished and they became brittle. Apparently hardness is not a guide to the oxygen content of chromium metal.
It has been postulated that even a minute quantity of nitrogen in chromium collects at grain boundaries as chromium nitride and produces brittleness.


If this is true, then crystalline chromium produced in a salt bath probably picks up its nitrogen as a surface impurity upon exposure to the air. When the metal is melted, this becomes disseminated throughout the entire test button or ingot, and ductility disappears.
Conclusions
Chromium may be refined in electrolytic cells with fused sodium chloride- chromium chloride electrolytes with a large degree of beneficiation with respect to nitrogen and oxygen content. With a feed containing 0.03 pct. nitrogen and 0.4 pct. oxygen, reductions of between 15 and 30 to 1 were obtained for nitrogen, and between 12 and 60 to 1 for oxygen. Sulfur content can be reduced. With feeds less pure than were used here, a reduction in nearly all contamination, especially by metals considerably more electropositive or
electronegative than chromium, can be expected. If cell liners are iron free and nonporous, a reduction of 2:1 in iron content can be obtained. A nickel-lined cell produced chromium containing 0.24 pct. iron from a feed containing 0.55 pct. iron. However, the product was contaminated with nickel.
The operating parameters of voltage, current, bath strength, and operating temperatures are not critical. Baths containing 3 to 10 pct, chromium and voltages of 0.5 to 1.5 are suitable in point of production rate and product quality. The permissible current is probably limited by the effective anode area. A cell of 10 in. inside diameter and with a coarsely fragmented anode feed covering its bottom yielded bright metal at over 100 a. at a production rate of over 80 g./hr. A current of 150 a. yielded a muddy grey product. The effect upon analysis is not known, but other similarly refined metals, notably titanium and vanadium, suffer in analysis and in physical qualities when voltage and current are high enough to degrade their appearance. The lower limit on temperature is set by the freezing point of the bath, and the upper limit by considerations of operating economy and of increases in the salt-sublimation nuisance.
