Table of Contents
I want to emphasize the point brought out: if solutions are absolutely pure, the tank-house troubles are very few. If you have one part in two million of antimony, you will have trouble, and lots of it, in the cell room; that is about the gage you might take of the degree of purity to which the electrolyte must be brought. Our investigations have not been exhaustive, as the plant with which I am connected is being dismantled. When we knew we were going to dismantle, we ran a good many tests, doping the commercial cells with impurities, such as arsenic, cobalt, and nickel. Cobalt is very injurious and must be kept very low.
I should like to call attention to one more matter, Leaching processes generally are assuming an increased importance, and they are sure to become much more important than they have been in the past. We have in this country large deposits of low-grade copper ores, such as that at Ajo. In general the surface portions of the ore-body are oxidized, the underlying ores are the original sulfides, and the intermediate ores contain both sulfide and oxidized copper minerals. So far leaching has been confined to the treatment of the oxidized surface ores with sulfuric acid. This treatment recovers little or none of the sulfide copper. On the other hand, present concentration methods applied to the mixed ores recover but little of the oxidized copper. It is therefore proposed to crush the mixed ore, leach out the oxidized copper, grind the leached tailings, and recover the sulfides by flotation. I believe that there is an excellent chance that we can make an 85 per cent, extraction of all of the copper in mixed ores of this character by roasting and leaching them, and that the operating costs for such a process will not be as great as the costs of leaching followed by flotation. The development of such a process depends very largely upon working out proper roasting methods. It seems to me that the small electrically heated roaster which was used for the zinc-roasting experiments points to a readily available tool for exploring the copper-roasting problem.
There is a difference in the results obtained in roasting in a muffle, or single-hearth furnace, compared with results obtained when the hot ore drops from one hearth to another through a current of hot gas, as in the case of McDougall, Herreshoff, and Wedge furnaces. When dropping through the hot gases, there is an intensified action on the small particles, which flash up at an intense heat while the mass and relatively smaller surface exposure holds down the heat in the larger particle. Where iron is in close contact with the zinc, the tendency to form ferrite is greater in the very fine material, while the tendency to have unroasted zinc increases with the size of the particle.
In the grinding of ore in a dry tube mill without air separation, there is a marked difference in the proportions of the various sizes from the product obtained by wet grinding in closed circuit with a Dorr classifier. It is necessary to exercise good judgment in deciding the best procedure for grinding. It is not correct to screen out the various sizes of the leached product and have these analyzed and then assume that the size which contains the higher per cent, of zinc is yielding less zinc. In some cases I found that the finest particles from the leached product ran high in zinc; but this was partly due to the fact that the finest material in the roasted product ran relatively high in zinc.
In all roasting operations, sizing of the particles is of the greatest importance. This is especially true of roasting mixtures of iron and zinc sulfides for zinc leaching, because the unroasted sulfide of zinc is not dissolved; and if ferrate of zinc is formed, each unit of iron renders 0.58 unit of zinc insoluble.
As with many other new processes, certain advantages have erroneously been claimed for the electrolytic zinc process, erroneous at least in degree. In particular, it has been claimed that low zinc content ores can be advantageously treated directly by roasting and leaching. Many of the ores in this class are ferruginous; the majority of them can be concentrated by old and well established methods; and, in my opinion, concentration ahead of roasting and leaching will generally make for a better economic return than the direct-roasting and leaching of the original total ore bulk. In either case, the ore must be crushed and ground; the concentrator expense, aside from the grinding costs, will generally be less than the roasting cost, per ton of material handled; the cost of roasters alone, when treating the entire ore bulk, will generally be greater than the combined cost of concentrator and roasters if the ratio of concentration materially exceeds 3:1. The extraction of zinc will ordinarily be as great for the combination of concentrator and zinc plant as it will be for the zinc plant alone, while the zinc plant costs will be diminished in many respects in proportion as the tonnage requiring treatment is diminished. In the west, practically all of the zinc ores carry valuable byproducts—gold, silver, lead, copper. These byproducts become available only through smelting processes. Their concentration in the original ore is seldom such as will permit sending the leach-plant residues to the smelter, while their concentration in the leach-plant residues resulting from the milled product will generally make it, profitable to smelt those residues.
I have noticed that, where ferrites are troublesome in the roasting of zinc ores, the extraction is usually very much lower than where they are not supposed to be present. I think that ferrites sometimes are blamed for some unroasted zinc for the reason that when ferrites do form, the temperature of roasting is usually maintained somewhat lower than is allowed where iron is not present in a form that produces ferrites. Grading the ore will, therefore, allow us to get the highest temperature conducive to good roasting without the formation of ferrites.
Mr. Hansen says that when roasting a ferruginous blend, it is much easier to form sulfate of zinc. It seems to me that this is fairly easily explained from the fact that iron oxide is the contact material very often used for making sulfuric acid, and from that angle, it seems very reasonable that marmatite ores should allow us to form a greater proportion of the zinc sulfate during roasting. I feel that marmatites are perhaps blamed for more than they might be. In my experience, the most extreme case of iron sulfide in solid solution with zinc sulfide—which constitutes marmatite —contained about 14 per cent of iron in the purest piece of the mineral that could be picked from the ore and was almost coal black. The same piece of mineral ore would contain, say, 54 per cent, of zinc.
The formula for ferrite of zinc would only call for about the equivalent weight of zinc on iron which, in this case, would be, say, 15 per cent, of zinc. In other words, if ferrite were the explanation of low extraction of zinc during leaching, the iron in that ore should have held back 15/54 of the total zinc. Actually, it was impossible to roast the ore to leave only that amount of zinc behind, and you could always find some unroasted blende on grinding and mechanical separation in the laboratory, which seems to say that the formation of ferrites makes it impossible for us to roast it at a temperature which will allow complete desulfurization.
The Anaconda Mining Co. has a plant that produces daily 150 tons of electrolytic zinc. The Consolidated Mining and Smelting Co. of Canada has a plant at Trail, B. C., that was producing regularly 30 tons of electrolytic zinc per day, but during the war the capacity was increased to double that. In Australia, they built one plant to produce 10 tons a day; that plant is so successful that they are going to increase it immediately to a capacity of 100 tons a day. There were several small plants in the United States. One was producing 7 to 8 tons of zinc per day. The war prices for high-grade zinc, of course, assisted a good deal in the development of the process. Now that the war prices are being modified and the demand for zinc temporarily lessened, the work is greatly curtailed.
Dorsey A. Lyon, Washington, D. C.—As Mr. Hansen has brought out, we must look to hydrometallurgical processes to treat our ores. I believe that the day of the old-line treatment of those western ores is past. We will have to find a treatment for ore where we are far away from railroads, and under desert conditions, where we have no water, or have to contend with one kind of fuel; the electrolytic zinc is just one phase. Notwithstanding the great progress of the last few years, there will be greater progress in the treatment of these ores in the next decade.
C. A. Hansen.—It has been said that the electrolytic zinc process is not a poor man’s process. The criticism is a valid one. Aside from the cost of the primary power station, the cost of a completely equipped electrolytic zinc plant may be taken at from $30,000 to $20,000 per ton per day of zinc capacity, as compared, for example, with something like $35,000 or $40,000 per ton of copper per day capacity for a large smelter and refinery treating fairly rich copper concentrates. I believe that, in respect to plant cost, the electrolytic zinc plant compares favorably with that of the alternative retort plant, whatever the scale of operations.
G. D. Scholl.—The plant at Keokuk was small, about 10 tons a day, but it was very successful. It is not being dismantled on account of failure but because it was decided not to build a commercial plant at this time. We had a peculiar plant; we treated fume entirely. A combination of fume and concentrates would be very good. We did not have time enough to develop as much of that work as we wished, but we did do a little with a combination of roasted Joplin ore and fume. The great difficulty in electrolytic zinc is the impurities. It is necessary to work to a very close limit and if roasted ore is combined with the fume, it is possible to dilute the impurities that come from the fume, and then by liberating certain portions of the solution every day, a happy medium may be obtained. There is much work to be done along these hires.
J. W. Richards, South Bethlehem, Pa.—I do not know exactly why the metallurgy of zinc in America has run toward the electrolytic method and in Europe toward the electrothermal. At present four plants in Norway, Sweden, and Finland are working on zinc ore by the electrothermal process and they are apparently prosperous. They have not taken up electrolytic methods at all. The reason may be their very cheap power. A furnace in southern Norway of 1000 kw. capacity is working on distilling zinc from zinc dross, possibly dross brought over from Germany. That furnace is operating with about 2000 kw.-hr. per ton, distilling the zinc at a cost of about $12 a ton. A very interesting side business was the distilling of an alloy containing 9 per cent, zinc and 4 per cent, copper, which was used for some purpose in Germany during the war. The zinc distilled over, leaving the aluminium-copper alloy, with lead and iron, for which they had found no use. Electric furnaces for working ore and distilling zinc have found a permanent place in the Scandinavian countries.
C. H. Fulton—Are any details as to the type of furnace used in Norway, Sweden and Finland available?
J. W. Richards.—At the present time, the companies are extremely secretive. They say they are working under the De Laval patents or some modification of them but will give no information as to the form of the furnace.
O. C. Ralston—It seems to me that the electrothermic extraction of zinc, when we know better how to operate it, will probably be better adapted to the small pocketbook. Mr. Allmand describes a zinc distilling plant built during the war, or just before, in the Cologne area, which he investigated for the British Government. He describes a large, cylindrical furnace, 15 ft. in diameter by 15 ft. long with four electrodes at each end, and a smaller condenser with its end abutting on to the furnace proper. It was stated that a 77 per cent, recovery of zinc was made in this furnace during the war, with a zinc fume and zinc oxide charge, containing over 70 per cent. zinc. Until this recovery is improved, I do not think this furnace will compete with ordinary thermic zinc, but it seems to be a very promising kind of furnace and does not involve briqueting, with its endless troubles. Incidentally, the expense would be lower, as no particular preparation of the charge is necessary, whereas one of the papers read today describes an electrothermic zinc extraction process in which a highly involved briqueting, calcining, and handling treatment is used. These recent developments in electric zinc distilling lead me to feel that economic units of small capacity, united to the size of small pocketbooks, will soon be possible.
Chairman Mathewson.—I would like to modify the last speaker’s statements to some extent. In the first place, the power required in the electrothermic treatment is about two and a half to three times as much as that for the electrolytic, so that power must be extremely cheap for that process to compete with the electrolytic method. Further, it has been found that for successful electrothermic treatment, a preliminary treatment must be given to the ore.
C. H. Fulton—What power is required for electrolytic zinc?
Chairman Mathewson.—The power is calculated on the zinc produced from the solution; you can easily average 10 lb. per kilowatt-day by the electrolytic method, but only 4 lb. with the best electrothermic treatment. For days at a time, considerably over 12 lb. per kilowatt-day has been produced in some of the electrolytic plants.
C. A. Hansen.—We have consistently made zinc under commercial operating conditions, but at current densities approximating 10 amp. per sq. ft., with as little as 2500 kw.-hr. direct current per ton of zinc cathodes. This, however, is not good economy by reason of the excessive cost of the low-current density electrolytic plant; 3000 kw.-hr. direct current per ton of cathodes is a perfectly practicable figure. The total power consumption for a reasonably large plant should not exceed 4000 kw.-hr. high-tension alternating current per ton of spelter actually shipped, this figure covering all power consumed in the zinc plant but not including power required for concentration.
Professor Pulton asked for power consumption reduced to the ton of 60-per cent, concentrates. It is right here that the basic difference between the retort process, or electrothermic process, and the electrolytic process comes in. The power consumption for the latter plant is practically independent of the grade of ore treated, and it is virtually 4000 kw.-hr. per ton of zinc output regardless as to whether that zinc is made from 60-per cent, zinc concentrates or from 25-per cent, zinc ores. On the other hand, with the thermic processes, the power consumption, the coal consumption, and in fact the costs as a whole, are practically the same for a ton of material treated, without regard to the zinc available from that ton of material.
George A. Guess, Toronto, Can. (written discussion).—On studying Mr. Hansen’s figures I noticed the very high percentage of heat in his roaster gas, so having made balance sheets on roasters in which the figures were considerably less, I recalculated Mr. Hansen’s problem. It is my opinion that the safest way to make a balance sheet is to actually calculate the heat in the gases and then leave the heat unaccounted for to heat lost by conduction, as figures for conduction and radiation are not very reliable.
Nearly every man has his own way of stating a heat balance. On smelter work I have always used the ton calorie, when weights were, in tons of 2000 lb. The relation between cubic feet and ounces being the same as between cubic meters and kilograms simplifies calculations in English units.
My finding is that only 61.5 per cent, of the heat generated in the roaster is carried away in the furnace gases; this figure also compares with my previous experience.


At normal temperature and pressure the above weights would correspond to the following volumes in cubic feet.

The only determined SO2 in his furnace gas was 2 per cent, in test No. 3. Taking this figure, the total volume of gas will be 8,410,000 cu. ft. per day, and deducting the above total gives 7,874,100 cu. ft. per day of oxygen and nitrogen.

For conduction losses, I have taken a difference in temperature of 650° C., 18 in. of firebrick, K value 0.0042, which gives a heat loss for the furnace per minute of 22,000 pound-calories or per day 15,840 ton-calories. My heat balance then would be as follows

C. A. Hansen (author’s reply to discussion).—I separated various screen portions and leached these separately. Each portion was analyzed both before and after leaching and I believe the conclusions reached regarding the relations between screen size, extraction, etc., are correctly reported. I feel, then, that I should escape Mr. Hamilton’s criticism in this respect.
I agree with Mr. Ralston that the presence of iron in zinc concentrates probably has much to do with the formation of sulfate zinc. I tried to develop some definite relation between iron content and sulfate formation, but I did not succeed in finding a relationship that would stand my analysis.
There are several reasons for not accepting so simple a relationship as that which Mr Ralston proposes. Perhaps the most obvious is suggested by the behavior of two such dissimilar materials as the Bully Hill ore and the Butte Superior flotation concentrate during the latter stages of the roasting period. It is not impossible to roast a high iron-zinc concentrate, whether marmatite or otherwise, so as to eliminate practically all sulfide sulfur. The average sulfide-sulfur content of the calcines obtained at Bully Hill was not higher than 0.1 per cent, and it was often lower. Mr. Ralston may have tried to roast a coarse concentrate or to roast too quickly. I do not in the least doubt that zinc concentrates are often incompletely roasted in commercial practice, and that insoluble sulfide zinc is frequently charged improperly as ferrate zinc; but I do not agree that the presence of considerable iron will cause one to choose between ferrate formation and incomplete desulfurization.
I thoroughly agree with Mr. Guess that it would have been preferable to determine the volume of the roaster gases, and their heat content, in making up a roaster heat balance. Both my own heat balance, and that of Mr. Guess were, unfortunately ex post facto balances. Either could undoubtedly be much improved were the data collected with definite regard to composing a proper balance.
At the time my data were collected, we were trying to get a new plant into production and we naturally compromised considerably between expediency and thoroughness. We had no proper facilities for measuring gas volumes nor for accurately sampling the roaster gas. In fact, the reported sulfur-dioxide content meant little more to us than that it showed an ample supply of oxygen. On the other hand, I have considerable data covering the rate of heat dissipation of various surfaces maintained at various temperatures—data collected in connection with the design of electrically heated apparatus.
It was comparatively easy to measure the surface temperatures at various points on the roaster shell. Whatever inaccuracies may be found in the reported balance sheet I doubt if the surface heat losses will be found very much in error. I am sorry that my files are not available since it might have been of interest to tabulate some of the data relative to surface temperatures versus surface heat losses. However, I think that some of these data have been published by Doctor Langmuir and by C. P. Randolph in the Transactions of the American Electro-chemical Society.

Electrolytic Zinc Plant
The electrolytic zinc plant of the American Zinc Co. of Illinois was described by Davidson in 1944. Since then, improvements as well as expansion of the plant facilities have been made. In order to increase the production of high grade zinc which was needed for war purposes, an expansion program designed to double the slab zinc capacity was started in 1942 and completed in March 1943. This expansion was propagated by a contract between the American Zinc Co. of Ill. and the Defense Plant Corp. The contract included the facilities of the Fairmont City, Ill., property of the American Zinc Co., where a suspension-type roaster with contact acid plant, cadmium distillation furnace, Waelz oxide and densifying plant, and horizontal retort furnaces were installed.
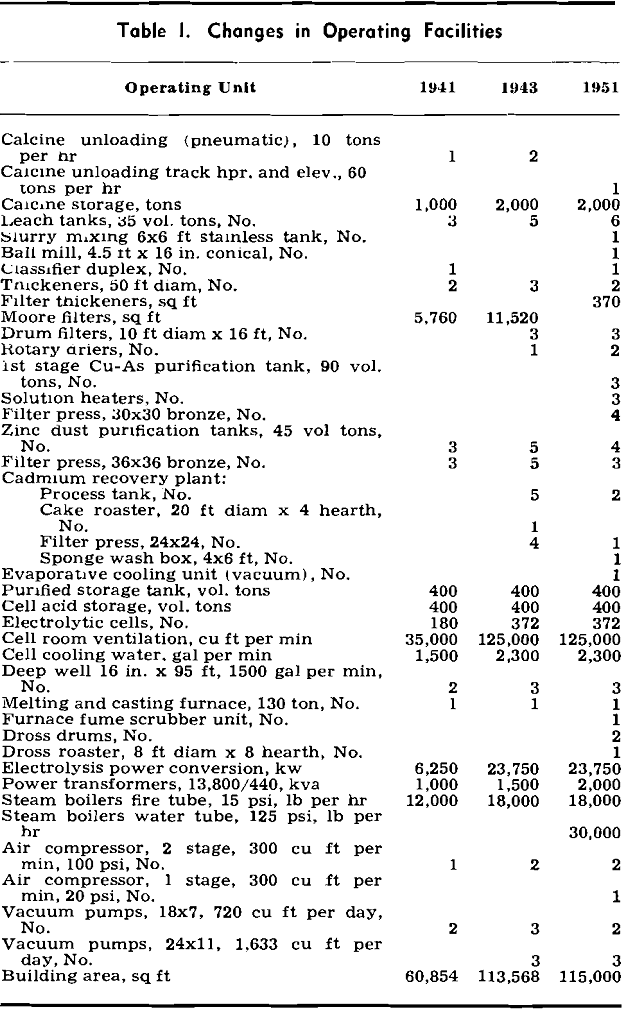
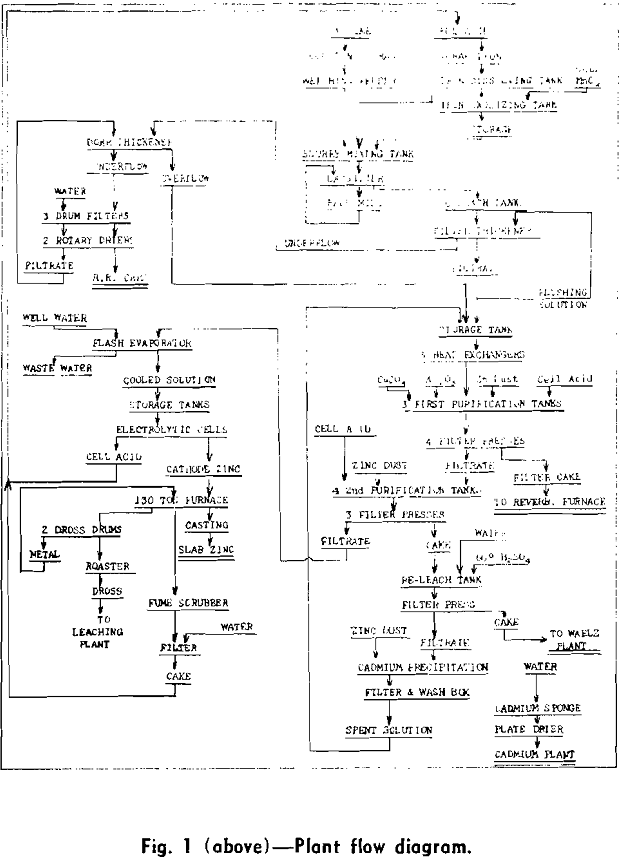
Leaching
Briefly, the leaching operation consists of the addition of spent electrolyte, about 130 g H2SO4 per liter, to a basic slurry prepared from spent electrolyte and calcine. The slurry, averaging about 1.45 sp gr, is prepared in a slurry mixing tank, Fig. 3, coupled with a classifier and ball mill. The main leaching operation is carried out in six lead and acid-proof-brick-lined tanks of 35 vol. ton capacity (a volume ton is equivalent to 240 gal). Maintaining the leach in a basic condition through most of the leaching cycle eliminates the settling and filtration difficulties encountered when calcines high in soluble silicates are leached in an acid condition.
Prior to the installation of the Cu-As purification step, the purifying effect of ferric hydroxide was of major importance in the production of solutions capable of electrolysis at reasonable current efficiencies. Ferric sulphate solution was made by dissolving scrap iron with spent electrolyte in eight lead-lined concrete cells of 3 vol. ton capacity and oxidizing the resulting ferrous sulphate with manganese dioxide (as manganese ore) in a wood stave, lead-lined tank.
Clarification and Residue Treatment
Prior to the installation of the additional purification step in 1951, clarification of the leach liquor was carried out in three 50 ft Dorr thickeners. Test-plant work indicated that the feed to the Cu-As purification step should not exceed 0.1 g per liter solids, which seemed impossible to attain consistently with the existing Dorr thickeners. The filtration cycle (filtration, flush back, and oscillation) is very short (35 sec) in order to take full advantage of the high initial filtration rate. Feed to the units averages 30 g per liter solids; the filtrate, 0.1 g per liter solids; and the underflow, 500 g per liter solids.
The third Dorr thickener is used as a residue washing thickener ahead of the rotary drum filters. The underflow from the filter thickeners is pumped into the washing thickener by air-actuated diaphragm pumps. Filtrate from the rotary drum filters, floor wash, and make-up water are also pumped into this thickener.
Purification
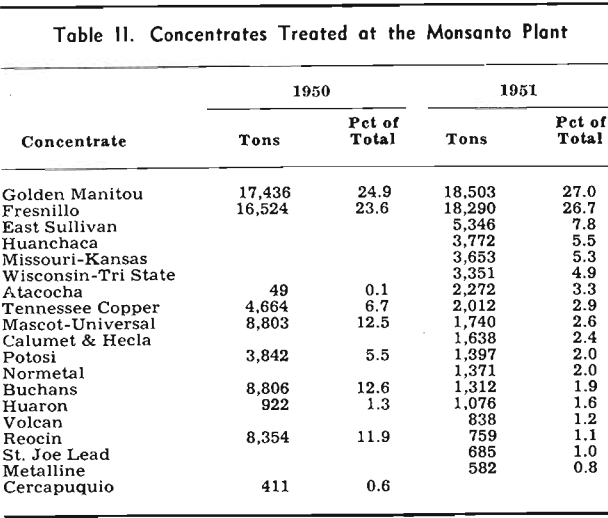
The purification process until 1951 consisted of the addition of zinc dust, copper sulphate, and potassium antimony tartrate to the clarified leach discharge liquor in order to remove the cadmium, copper, and cobalt. This procedure was adequate to deal with calcines whose solutions contained under 5 or 6 mg of cobalt; however, when calcines yielding greater concentrations of cobalt were treated, a serious reduction in current efficiency resulted. The cobalt content of the calcine therefore imposed limitations on the choice of concentrates for the Monsanto plant and made necessary a search for a more effective purification.
About 27 pct of the feed during 1951 came from concentrates which could not have been successfully treated before the installation of the Cu-As purification. As a temperature of from 90° to 95°C was found to be the best for this purification, it was necessary to install heat exchangers, to raise the temperature of the clarified leach liquors, which was about 65 °C, to this temperature.
The procedure for the addition of reagents is as follows: The copper sulphate in crystal form and the arsenic trioxide in powder form is added near the beginning of the filling cycle. The usual additions are 250 lb of copper sulphate crystals and 4 lb of arsenic trioxide. When the tank is about two thirds full, about two thirds of the zinc dust is added and when the tank is completely full, the remainder of the zinc dust is added. The average addition is 400 lb of zinc dust.
The extreme toxicity of the arsine gas generated in this process has been a matter of grave concern. This department, although included in the main plant building, has been isolated from other departments by a wall which extends from basement to roof. The room used for storing the press cake from this process is also individually walled off and exhausted by two 12,000 cu ft per min blowers. As stated previously, the tanks are vented by blower assisted stacks capable of moving 1700 cu ft per min.
The solution from the first purification stage is pumped through four Shriver 30 in. bronze plate and frame presses equipped with individual hydraulic closers. The press cake is dropped through openings under the presses into the press cake storage room beneath. At three week intervals the stored press cake is loaded into trucks by a gasoline powered scoop truck and hauled to the Fairmont City plant.
The zinc dust addition in this purification step averages about 175 lb per tank and is a more or less standard addition not subject to variation from tank to tank except on rare occasions. The solution is acidulated with spent electrolyte before discharging. The average tank purification cycle including filling and discharging takes about 3 hr. Tanks must be cleaned at least once a month because of the rapid accumulation of metallic zinc and cadmium sponge on the bottom of the tank.
Spent electrolyte is returned to cell acid storage sump by covered lead-lined wooden launders at the foot of the cascades.
Anodes are ¼ in. rolled and perforated 1 pct Ag- Pb alloy sheets. These are burned to chemical lead header bars cast around a copper bar. The lower edge of one end of the bar is exposed for bus bar contact. The sheet perforations are 3/8 in. sq and spaced on 7/8 in. centers.
Nine adjacent cathodes are lifted from the cells at one time by a ½-ton chain hoist hung on a strain insulator. Transport to the stripping floor is on an overhead monorail.
Cell Room Operation
Rated cathode zinc production is 128 tons daily. The average current is 11,800 amp. These meet ASTM special high grade zinc specifications.
Electrolysis is carried on at an average cathode current density of 36.5 amp per sq ft. The electrolyzing solution contains about 135 g per liter of zinc and is regulated to the upper ten cells of each cascade to maintain an acid concentration between 105 and 110 g per liter sulphuric acid. The tail cells are stripping cells and the spent electrolyte leaving them contains about 125 to 135 g per liter sulphuric acid. Manganese depletion in the cells is about 0.5 g per liter. Cell temperature is normally maintained below 40 °C. Acid concentration in each cell is tested nine times daily by measuring the conductivity of the solution.
Weighed cathodes with an estimated 0.5 pct moisture are melted in a 130 ton suspended arch, natural gas fired, reverberatory furnace.
Casting Operation
There are two types of slabs produced at Monsanto: the familiar 60 lb slab and an interlocking slab of the same size. Until 1947, the overall slab dimensions were 18 5/8×8¾x1½ in. and the slabs weighed 52 lb. In order to comply with the method of the Association of American Railroads for loading, bracing, and blocking carload shipments of zinc slabs, the overall dimensions of the slab were changed to 17¼x8 5/8×1 5/8 in. The slab weighed 60 lb. This change eliminated the need for longitudinal or center gate bracing, since the reduced slab dimensions would permit loading the car with six slabs the long dimension across the car for almost all box cars available at the Monsanto shipping point. The use of cast pallet slabs was also adopted at this time.

