Table of Contents
The objective of this investigation was to reduce toxic Cr6+ in various waste processing liquors to less toxic Cr3+ while simultaneously removing copper. In addition to recovering a valuable copper product, removal of copper and reduction of Cr6+ in these liquors will substantially decrease the chemical demand and increase efficiency in waste treatment operations associated with metallurgical processing.
Solutions containing Cr6+ (as chromic acid or sodium dichromate) and sulfuric acid are used in brass finishing, printed-circuit-board etching, preparation of plastics for plating, anodizing, and various other surface treatments. As the solutions are used, Cr6+ is reduced to Cr3+, the dissolved-solids content increases, and the acid concentration is reduced. The action of this etchant on a copper substrate can be represented by the following equation:
Cr2O2- 7 + 3 Cu + 14 H+ → 2 Cr3+ + 3 Cu2+ + 7 H2O
The solutions are discarded when the reaction rate is below what is required for a given operation. At this point, a spent printed-circuit-board etchant, for example, would contain considerable amounts of Cu2+, Cr3+, and Cr6+. A spent brass etchant would contain zinc in addition to these components. Technology for ultimate disposal of these wastes usually involves the reduction of the remaining Cr6+ to Cr3+, followed by base addition to precipitate chromium and other metallic hydroxides. The resulting sludges are then used for landfill.
The disposal of these wastes in this manner is cause for environmental concern, and it wastes valuable resources. Current Environmental Protection Agency (EPA) regulations prohibit sewering of waste water containing more than 0.25 ppm Cr6+ and 4.6 ppm Cu3+ by plants discharging more than 38,000 l/day. Although the value of the copper recovered from these wastes is not particularly great at current prices, its recovery would save costs associated with its disposal. Landfill areas suitable for metal hydroxide sludges are becoming scarce, and collection, treatment, and disposal by waste contractors are expensive. Further, removal of copper from these wastes would make them more amenable to recovery and recycling.
Cochran and George have shown that copper can be electrowon from spent brass etchant after all the chromium has been reduced. Several researchers successfully electrowon copper from dilute solutions using either porous electrodes or electrodes composed of small discrete particles. Bennion and Newman used porous carbon anodes and cathodes to reduce the copper content of solution from 667 ppm to less than 1 ppm. Chu, Fleischmann, and Hills and Paulin, Hutin, and Coeuret reported similar results. Kennecott Copper Corp. has operated a 100-lb/day prototype unit for recovering copper from dilute leach solutions. The unit uses thin particulate coke cathodes.
Copper has also been electrowon from dilute solutions using fluid-bed cathodes composed of either copper spheres or chopped copper wire. Resource Control, Inc., has designed a system to treat rinse water contaminated with electroplating wastes. The system uses a bed of “semiconductive carbonaceous material” between a standard anode and cathode.
The system described in this report uses an electrolytic cell having coke anodes and cathodes. Spent brass and printed-circuit-board etchants are fed into the cell where Cr6+ is reduced to Cr3+ and copper is electrowon. This process differs from the prior art in that Cr6+ is reduced, and copper is electrowon from concentrated wastes in a single step using granulated coke electrodes. Granulated coke electrodes are inexpensive, inert, and readily available. The coke electrode, with the reduced copper metal, can be sold for its secondary copper value or furnaced directly to produce a copper ingot.
Experimental Procedures
Spent Etchants
The compositions of the spent etchants used are given in table 1. Solution A is a spent printed-circuit-board etchant. Solution B is a spent brass etchant. Originally, both etchants were prepared from sodium dichromate and sulfuric acid. The compositions of etchants A and B are typical of solutions, in the application indicated, when they are ready to be discarded. The pH of these solutions ranges from 1.1 to 1.3. The compositions given are not to be regarded as representative of the exact point at which the solutions are discarded. The indicator of when the solutions are spent is often visual examination of the bath or product and not a chemical analysis.

Coke Electrode Cells
Small Coke Electrode Cell
In the initial experiments, a small semicylindrical electrolytic cell containing one anode and one cathode was used (fig. 1). The cell was fabricated from Plexiglas and contained five compartments separated by four polypropylene screens that contained the petroleum coke (minus 8- plus 16- mesh) . It is conceptually similar to the large coke-electrode cell described later except that it contains only one pair of electrodes. A strip of lead was used as the anode current carrier. Electrical connection to the cathode was made using either nickel or copper strip. In some experiments, the coke was contained in a stainless steel basket that was then placed between a set of polypropylene screen dividers. The stainless steel basket served as the current carrier. This arrangement reduced corrosion of the current carriers at the electrolyte-air interface. The method of electrical connection did not affect the cathode efficiency.

The current density was 2.15 amp/dm². (The area was calculated as if the electrodes were solid. Only the electrode area directly opposite another electrode was counted.) The actual current in the small cell was 2.7 amp at 2.7 to 2.5 volts.

The current was kept constant throughout the operation of the cell. The voltage remained fairly constant throughout the trials.
The cell held 650 ml of solution. An additional 250 ml was kept in a separate container and circulated through the cell at a rate of 10 ml/min using a peristaltic pump.
Large Coke Electrode Cell
This cell was constructed from a 30- by 30- by 30-cm polypropylene tank and held two anodes and two cathodes (fig. 2). Economical metallurgical coke (minus 8- plus 16-mesh) was held in four 30- by 30- by 1.9-cm holders fitted with polypropylene screen. Platinum strips embedded in the coke anodes served as current carriers; copper strips embedded in the cathodes served the same purpose. The electrode holders fitted snugly against the sides of the tank.
The current density in the large cell was 2. 15 amp/ dm². (This quantity was calculated in the same way as for the small cell.) The current was 42 amp at between -6and 4 volts. Voltage decreased as the operation proceeded. This cell is shown in figure 3.
The operating volume of the cell was 20 liters. A variable-flow positive-displacement pump was used for electrolyte circulation. When the large cell was operated in a continuous fashion, a peristaltic pump was used to feed spent etchant. The treated etchant was allowed to overflow the cell.

Results and Discussion
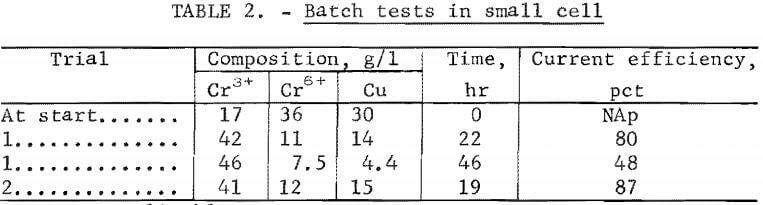
The reduction of Cr6+ to Cr3+ and copper electrowinning were achieved with both the spent printed-circuit-board etchant and the spent brass etchant. Significant pH changes were not observed during the electrolysis. Table 2 gives the results of batch treatment of the spent printed-circuit-board etchant in the small cell. Efficiencies were calculated on the basis of total equivalents of copper and Cr3+ produced. These data indicate that simultaneous Cr6+ reduction to Cr3+ and copper deposition occurred with fairly good efficiencies during the initial -45 pct of the trial. There were two major problems with this cell: severe corrosion of the current carriers and short-circuiting. Anode and cathode surfaces were within 1.3 cm of each other. Growth of dendritic copper on the electrode surface usually short-circuited the cell after about 48 hours.
The large cell was constructed to correct these problems. Electrode spacing was 4 cm (surface of the cathode to surface of the anode). Platinum strips embedded in the coke anodes and copper strips in the cathodes were used as current carriers, which solved the corrosion problem. Table 3 gives the results of batch tests in the large cell at various circulation rates. Initially, efficiencies appear to be better at the lower circulation rates; however, dendritic copper growth on the cathode is less severe at higher rates. In 48 hours, only 0.02 g/l Cu or less and no detectable amount of Cr6+ remained in the treated etchant in all of the tests. Variance in the amount of Cr3+ and zinc in the solution is the result of volume changes during the electrolysis. (Water was added periodically to make up evaporation losses.) No short- circuiting problems were encountered during the batch tests.

The results of continuous operation of the large cell are presented in table 4. Twenty liters of spent brass etchant was placed into the cell and electrolyzed for 24 hours. Spent etchant was then fed into the cell at 14 ml/min, and treated etchant was removed through an overflow tube located near the top of the cell. Etchant in the cell was agitated by circulating the solution with a positive-displacement pump at 100 ml/min. The circulation was from anode to cathode.

The higher circulation rate was chosen to minimize dendritic copper formation and subsequent short-circuiting. No problems with short-circuiting were encountered during the trial, but an examination of the cathodes after the run showed a substantial buildup of copper metal on the polypropylene screens.
This copper buildup resulted in an increase in efficiency because the electrode was rendered more conductive.
Overall 81 pct of the copper was removed and 86 pct of the Cr6+ was reduced to Cr3+ at a current efficiency of 52 pct in the continuous run. The power requirement of copper electrowinning is about 6.6 kwhr/kg (3 kwhr/lb) for the spent brass etchant. This is essentially the same power requirement as Kennecott’s process for winning copper from dilute leach solutions.
It appeared that no Cr3+ was oxidized at the coke anode. Small anode weight losses were observed.
Electrolytic Reduction using Coke Electrodes
The reduction of Cr6+ while simultaneously removing copper from spent brass and printed-circuit-board etchants has been demonstrated. Using an electrolytic cell fitted with coke anodes and cathodes, 81 pct of the copper was removed from a spent brass etchant and 86 pct of the Cr was reduced to Cr3+ in continuous operation. The technique described has potential for simplifying the disposal of these waste etchants as well as for recovering a valuable copper product, although EPA limits for Cr6+ and Cu have not been achieved in this study.
Solutions containing Cr6+ and sulfuric acid are used in a number of surface-finishing operations. Substantial quantities of chromium and copper are lost and pollution problems are created when the spent solutions are discarded. To minimize the undesirable environmental effects while improving secondary resource recovery technology, the Bureau of Mines is conducting research to detoxify and recover metals from these solutions. Spent etchants are treated to remove copper and reduce Cr6+ to Cr3+ in an electrolytic cell. The anodes and cathodes are granulated coke. The spent solution is continuously pumped through the electrodes. Hexavalent chromium is reduced to trivalent chromium, and copper is recovered at the cathode. Reoxidation of Cr3+ at the anode was not observed. The removal and recovery of copper and reduction of Cr6+ lessen chemical demand and increase efficiency in waste treatment operations associated with the use of these solutions.
