Table of Contents
Electrolytic Method for Recycling Scrap Batteries Objective
To devise an economical, environmentally acceptable method for recycling scrap lead-acid batteries.
Approach
A combination electrorefining-electrowinning method for recycling lead metal and sludge from scrap batteries was devised which produces a 99.99 + percent pure lead product and eliminates the lead and sulfur oxide emissions that are the normal by products of high-temperature smelting processes.
How the Method Works
Lead metal grids and lugs are separated from the battery sludge by washing and screening, and are melted and cast into anodes for electrorefining by the Betts process. The electrolyte used is fluo-silicic acid, a large volume waste product generated during the production of phosphate fertilizer. During the electrorefining process, impurities in the anode are trapped and held in an adherent slime blanket on the surface as the anode dissolves while pure metallic lead is deposited on a lead cathode.
The major problem in the past has been to recover the lead from the battery sludge which consists of approximately 60 percent lead sulfate, 21 percent lead, and 19 percent lead dioxide. In the flow sheet developed by the Bureau
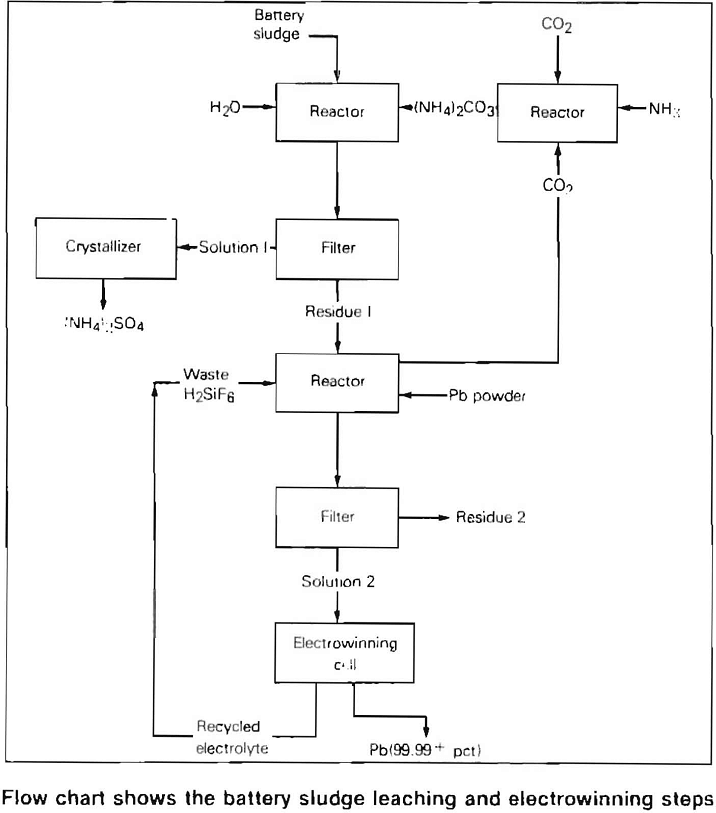
of Mines, the sludge is treated in a two-step leaching operation which produces a waste product of ammonium sulfate, usable in commercial fertilizer, and a lead fluosilicate solution which is used as the electrolyte during electrowinning. In the electrowinning step, the anode does not dissolve in the electrolyte, and lead is recovered or won from the solution. The insoluble lead dioxide-coated titanium anode patented by the Dept. of the Interior has proven to be outstanding for use in lead electrowinning.
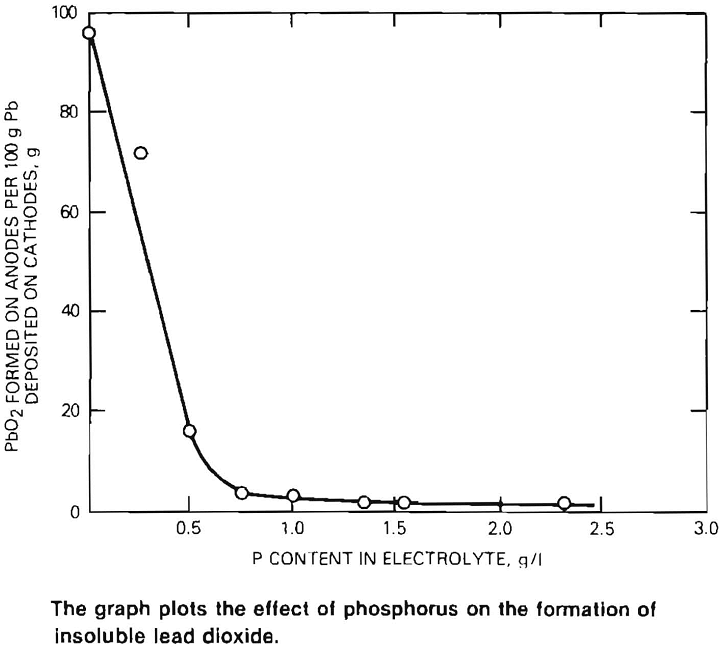
In the past, lead electrowinning has not been successful because much of the lead in the solution collected on the anodes as an insoluble lead dioxide compound at the expense of metallic lead deposition at the cathode. A major breakthrough was achieved with the discovery that lead dioxide formation is prevented by the addition of as little as 1.5 grams per liter of phosphorus to the electrolyte (see graph). This makes the waste fluosllicic acid particularly well suited for electrowinning since it already contains the necessary phosphorus (1.5 to 2.5 grams per liter).
Test Results
Laboratory-scale research using a 20-liter multielectrode cell is nearing completion. Lead of sufficient purity (99.99 + percent) for the manufacture of maintenance-free batteries was produced with minimal pollution. A particular advantage was the ability to use the waste fluosilicic acid as the electrolyte.
A preliminary economic evaluation indicated that, assuming a price for lead of 29 cents per pound, a small profit might be possible—about 7 percent return on investment. Since that evaluation was published, a major improvement has been made in the flow-sheet that should have a favor-able economic and environmental impact. This improvement involves using ammonium bisulfite instead of lead powder for reducing PbO2 in the sludge to soluble PbO.