Table of Contents
The theory of electrolysis cannot be taken up at any length here, and the student desiring fuller information on this subject is referred to the works of Ostwald, Nernst, James Walker, and others. Briefly, it may be stated that on passing a current of electricity through a solution of a salt in water the salt is broken up. This process is termed electrolysis. The conductors connected to the two battery terminals are called electrodes ; that connected to the zinc of an ordinary cell being termed the negative electrode or kathode, and that with the other terminal the positive electrode or anode.
The free positive and negative radicals produced by the action of water on salts are termed ions (things that go); e.g. on dissolving NaCl in water the sodium chloride is split up, if the solution be very dilute, into the ions Na and Cl the Na carrying a charge of positive, and the Cl a charge of negative electricity ; and the amount of electricity carried by the Na ion is equal to that carried by the Cl ion, but opposite in sign, hence the solution as a whole is electrically neutral.
But on introducing the electrodes and passing a current, one electrode is charged with positive electricity and the other with negative. The positive ion travels to the negative electrode and the negative ion to the positive electrode. The Cl ion, for instance, travels to the positive electrode or anode; there it gives up its negative charge, and two of the uncharged Cl ions unite to form the molecule Cl2, a gas. The Na ions with their positive charges travel to the kathode and are there relieved of their electrical charges. The metallic sodium thus formed at once reacts with the water present, forming NaHO. That is, the products of the electrolysis of a solution of NaCl are chlorine gas and caustic soda.
The ions charged with positive electricity are attracted by the negative electrode or kathode and are termed Kathions (Cathions). Hydrogen and the metals come under this head.
Those charged with negative electricity wander to the positive electrode or anode and are termed Anions. The acid radicals belong to this class.
It must be remembered that these ions existed in the solution before the current was passed; the current merely collects them at the electrodes according to their respective charges of electricity—whether positive or negative.
In conducting analysis by electrolysis the following points must be considered :—
1. Production of the Current.
2. Measurement of the Current Strength.
3. Measurement of the Tension.
4. The Electrodes and Electrolytic Cell.
Production of the Curren
—Various forms of cells, primary and secondary, and dynamos, may be used for the production of the current. For the student’s purpose, however, two quart Daniell cells will suffice. Each of these consists of a large glass jar containing a copper cylinder (or simply an outer copper cylinder) and filled with a saturated solution of sulphate of copper, the crystals being present in considerable excess. Inside the copper cylinder is placed a porous earthenware pot, containing dilute sulphuric acid, and a zinc rod, amalgamated liberally by rubbing under dilute acid with mercury. On immersing the zinc rod in the sulphuric acid (1 of 36E. H2SO4 to 12 parts water) it should not produce a brisk effervescence.
The two cells are connected up thus: the wire from the zinc of one cell is connected to the copper of the next. The two free terminals—a copper and a zinc—are connected by wires direct to the electrodes, the wire from the zinc being connected to the kathode and the wire from the copper to the anode.
For other methods of generating the current and other details concerning electrolytic analysis the student is referred to the excellent treatise of Drs Classen and Lob (translated by Herrick and Boltwood) on Quantitative Chemical Analysis by Electrolysis.
Measurement of the Current Strength
Just as in estimating the work done by water we consider the quantity of water and the head or pressure, so here we consider quantity and tension or pressure, the quantity of the current being measured in amperes and the tension (or pressure) in volts. The exact value of these units is given in any elementary text-book on electricity. Under this head the measurement of the current (or quantity of electricity) is considered.
In order that the student may know exactly the conditions under which he is working, the current should be measured by an ampere-meter (ammeter) with a range of 0 to 6 amperes. To check the current strength this instrument is introduced into the circuit and the quantity of electricity in amperes is read direct from the dial of the ammeter.
To reduce the current a resistance box may be introduced into the circuit, and to increase the current, if cells are used, a number may be connected in parallel, that is, all the zincs are connected together and all the coppers, and a leading wire is taken from each group to the electrodes.
The student is advised not to attempt to procure the current from electric mains, either power or lighting, unless acquainted with the properties of electricity, and the necessary adjustments required to regulate these strong currents to his requirements. (He may investigate the suitability or otherwise of alternating currents for electrolytic analysis.)
Measurement of the Tension
That this may be accurately ascertained, it is advisable that the student procure or obtain the use of a voltmeter such as Weston’s, which gives values accurate to 1/10th volt, the voltage being read direct from the dial.
To increase the tension with cells, connect up a number of them in series, that is, zinc to copper, zinc to copper, and so on. If at the same time it is desirable to increase the current strength or amperage, connection by series parallel may be resorted to. For example, 16 cells may be connected thus: Connect 4 cells in parallel, then other 4, and so on till 4 sets of 4 cells each in parallel are obtained. Then consider each set as a single cell, and connect the zinc of one set to the copper of the next and so on.
A convenient method of lowering the tension is by the introduction of a shunt circuit. If the two poles of a cell or other source of current be connected by a wire, and a loop of similar wire be connected to two points in this wire some distance apart, then part of the current flows through the main wire and part through the loop, and the tension in the loop is to the tension between the two terminals as the distance apart of the loop connections is to the length of the main connecting wire, assuming the wire of equal cross section and uniform throughout.
Electrodes & Electrolytic Cell
If special electrodes are not available the student may use a large platinum dish or crucible for the kathode, and for the anode a platinum wire, terminating somewhat as shown in fig. 70b (see next page). It is, however, recommended that the electrodes to be described be purchased or manufactured from thin sheet platinum and platinum wire.
The condition of the precipitate formed by electrolysis depends greatly on the strength of current flowing through the cell. A current of twice the strength will precipitate twice the quantity of metal in a given time. The strength of current therefore determines the rate of deposit on the electrode.
This being so, the size and shape of the electrodes become a matter of importance. If the area of an electrode is small and the current density high, the atoms of metal are deposited in such rapid succession, crowding together, that a coherent precipitate is not obtained; and as in electrolytic analysis the precipitate is generally to be weighed on the platinum electrode, a non-coherent precipitate cannot be expected to give good results. Again, with the opposite extreme—large electrode surface and low current strength —the coating will be patchy, and again unsatisfactory.
It is evident, then, that some mean between these extremes should give the best results. A convenient standard of measurement is that adopted by Dr Classen, the current density being always given with reference to 100 square centimetres of electrode surface. Thus, if a platinum cylinder 2.5 cm. x 7.5 cm. be used as the kathode, and dip 5 cm. in the solution to be electrolysed, then the surface available is 2.5 x 22/7 x 5 x 2 = 78.6 sq. cm. Suppose the ammeter shows that 1.2 amperes flow through the cell, then every 100 sq. cms. receive a current of 1.2 x 100/78.6 = 1.52 amperes. Classen adopts the symbol ND100 to represent the current density per 100 sq. cms. electrode surface. In the example, then, ND100= 1.52 amperes.
When determining metals it is sufficient to know the current density at the kathode alone, but when determining the halogens and other elements or compounds deposited on the anode, it is important that the current density at the anode be known. As a rule, a current density of ND100 = .5 to 1.5 amperes is most suitable, though exceptions on both extremes are met with. The tension, as a rule, runs from 1 to 4 volts.
The most convenient form of kathode is a plain platinum cylinder, as shown in fig. 70a.
Length, 7.5 cm.; diameter, 2.5 cm. ; length of connecting platinum wire, 12 cm. This wire is riveted to the cylinder, which is more convenient if cut down the side opposite to the wire. This allows of the easy removal of the kathode without previous removal of the anode. The weight of the kathode is about 15 to 18 gms.
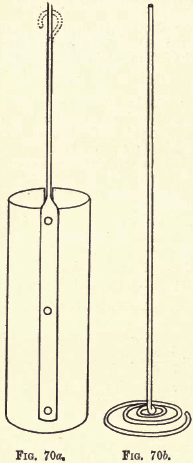
The anode is made of platinum wire about 1 to 1.5 mm. in diameter. Length of straight stem (see fig. 70b) is about 20 cm. At the base, as shown in the figure, this wire terminates in a coil about 3 cm. in diameter, the folds of the coil being spaced about 3 mm. apart. This form of anode ensures a uniform evolution of gas, and an even deposit on the kathode both inside and outside.
It is essential to success that the student exercise great care in keeping the surfaces of the electrodes clean, otherwise an even metallic coating cannot be obtained. The kathode surface must be perfectly free from any greasy matter. It is also essential that all metallic connections in the circuit be kept bright and clean, or the current may be weakened or broken.
For the support of the electrodes the two stands shown in the figure may be used, the anode being attached as shown to the one and the kathode to the other, the stands and electrodes being adjusted in the position shown. A single stand with suitable connection may be substituted, or one of the racks (for a number of assays) described in the works of Beringer or Peters (Copper Smelting). The wires from the battery are attached as shown in fig. 71.
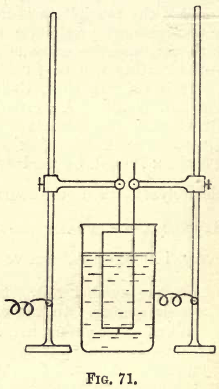
For the electrolytic bath a narrow deep glass beaker of 200 c.cs. to 250 c.cs. capacity is most suitable. With this form of beaker, if not filled more than two-thirds full, there is little danger of small particles of the solution being thrown over the edge by a rather brisk ebullition of gas.
Before proceeding to an analysis, the apparatus must be thoroughly cleaned, the necessary source of current prepared, and the apparatus connected up as follows:—Having charged two or three quart Daniell cells, connect them in series. If an ammeter and a voltmeter are available, the former may be connected to the free zinc terminal and the latter to the free copper terminal, and from each of instruments a wire is connected to the two standards shown in the last figure. The electrodes are then connected—the kathode to the zinc end of the battery—and the apparatus is ready for the electrolysis of the solution, which is placed in the bath described.
If the measuring instruments are not available, the free copper and zinc terminals are connected direct to the electrodes. Regarding the ammeter and voltmeter, it is advisable that they be removed, except when it is desired to measure the current. On removing the ammeter, its place in the circuit should be filled with a wire (or resistance roll) of the same resistance as the ammeter, otherwise the conditions of the analysis would be slightly different when the instrument was removed.
The voltmeter is best introduced into the circuit by joining a wire to the wire connected to the kathode and then to the voltmeter. The other binding screw of the voltmeter is then connected by a wire to the copper terminal of the battery, there being therefore two wires coming from the copper terminal and one from the zinc. All these connections should be made with wire at least 1.5 mm. in diameter.
ELECTROLYTIC ESTIMATION OF COPPER
(a) In pure copper sulphate.
(b) In a copper ore.
(a) IN PURE COPPER SULPHATE.
The apparatus described will suffice for this estimation. Good results may be obtained without the use of the ammeter, voltmeter, and resistance boxes, though it is advisable that the student should if possible ascertain definitely the conditions of the experiment.
For analysis, the student may take a sample of the pure sulphate CuSO4,5H2O previously prepared.
Method of Analysis
The copper sulphate is dissolved in distilled water, and nitric acid added in not greater quantity than 5% by volume. On diluting the solution sufficiently the copper sulphate is split up into the kathion Cu and the anion SO4. The discharged Cu is deposited on the kathode, and the discharged sulphate radical attacks the water thus:—
2SO4 + 2H2O = 2H2SO4 + O2
It must be remembered that on electrolysis HNO3 splits up thus—
2HN03 = H2 + NO3
and this hydrogen acts as a reducer on the HNO3 thus—
4H2 + HNO3 = NH3 + 3H2O
and in the presence of sulphuric acid or a sulphate the final product is ammonium sulphate.
On prolonged passage of the current, if iron or zinc be present, these salts would be broken up and their hydrated oxides deposited on the kathode.
The presence of chlorides must be avoided.
Best conditions of experiment—(Classen):—
Temperature, 20°-30° C.
Current density, ND100 = 0.5 to 1 ampere.
Electrode tension, 2.2 to 2.5 volts.
Volume of solution, for 1 gm. sulphate about 120 c.cs.
Time, 4 to 5 hours.
The Analysis
Weigh out 1 gm. of the sulphate which has been dried between sheets of filter paper. Transfer to a deep narrow 200 c.c. beaker. Add 100 c.cs. distilled water and 5 c.cs. 16E. HNO3. Make up to 120 c.cs. with distilled water.
See that the electrodes are thoroughly clean. Remove the kathode. Rinse with 5E. HNO3, then with distilled water, and finally with absolute alcohol. Gently ignite in a low bunsen flame till dry. Remove in the desiccator and weigh when cool. Replace the kathode. Bring up the beaker, raising it on a block till the electrodes dip about 6 to 7 cms. in the liquid. See that the anode does not touch the kathode, and is uniformly set with regard to it.
Allow the current to pass. A brisk stream of bubbles should rise from the coil and stem of the anode. If these do not appear or are very sluggish see that the battery cells are properly charged and that all connections are clean and properly made.
Continue passing the current for four or five hours, when the solution should be almost colourless. The end of the reaction is determined by removing about ½ c.c. of the solution by a pipette and testing in a small test tube with NH4HO or H2S (the latter can only be used if no other reacting elements are present), or lower the electrodes and notice if a fresh deposit forms. Draw off the solution with a pipette and wash down the kathode at the same time with distilled water. Fill a deep narrow 250 c.c. beaker with distilled water. Unclamp the kathode and remove it quickly to the water in the beaker. Remove the kathode from this beaker into another containing pure alcohol. Remove from the alcohol and gently heat in a low bunsen flame till dry.
Remove in the desiccator; cool and weigh. The difference between this weight and that of the kathode gives the weight of copper deposited. Compare the results of duplicates with those previously obtained.
Note.—To prepare the kathode for the next estimation, place in it a 250 c.c. beaker and add about 160 c.cs. 5E. HNO3 to remove the copper. Wash, dry, and weigh as before.
THE ESTIMATION OF COPPER IN ITS ORES
The necessary apparatus is the same as before. For analysis the student may take a sample of the ore previously used for the cyanide and iodide estimations of copper.
Method, Reactions.—After the preliminary treatment of the ore the copper is separated as before. If iron and zinc are present in the solution the current should not be passed longer than is necessary to effect the complete deposition of the copper, or the nitric acid present will be reduced and ammonia compounds formed, resulting in the deposition on the kathode of the hydrated oxides of iron and zinc. If the method to be described be closely followed the copper may be completely deposited from solutions containing iron, alumina, zinc, nickel, cobalt, chromium, manganese, cadmium, calcium, barium, strontium, and magnesium.
Mercury, silver and bismuth would, if present in the solution, be deposited along with the copper.
The Analysis.—Weigh out 1 gm. of the finely powdered and sampled ore. Transfer to a 250 c.c. beaker. Moisten with a little cold water. Add 25 c.cs. 16E. HNO3 and 10 to 15 drops 36E. H2SO4. Cover with a clock glass and heat on the hot plate till all the ore is in solution and the nitrous fumes have disappeared. Wash the clock glass into the beaker and evaporate the solution till dense white fumes of SO3 freely come off. The copper now exists as sulphate. Set aside to cool for a few minutes, and when sufficiently cool add, cautiously, 10 c.cs. 5E. HNO3, 4 drops 36E. H2SO4, and 40 c.cs. distilled water. Heat on the hot plate till the mass is in solution (neglect any insoluble matter). Filter off the insoluble matter
through a small filter. Wash thoroughly, keeping the bulk of the washings and solution below 120 c.cs.
As a precaution it is advisable to quickly dry and incinerate the filter and contents and treat the ash with 2 c.cs. 16E. HNO3. Evaporate in the porcelain crucible till nearly dry, and take up with 5 c.cs. water.
Filter through a 5 cm. filter. Wash once or twice and add the solution to the main solution. Any copper contained in globules of sulphur or in the filter paper is thus collected.
The solution is now electrolysed as before and the copper present estimated. Repeat the estimation. Duplicates should agree within .03% on ores running from 20% to 60% copper, and within .02% for ores containing less than 20% copper.
ELECTROLYTIC ESTIMATION OF COPPER & NICKEL IN COPPER NICKEL ALLOY
The apparatus necessary is the same as before. For analysis the student may procure a nickel coin or other alloy of copper and nickel.
Method, Reactions.—The copper and nickel are obtained in solution as sulphates, and from the acid solution the copper is precipitated as before. After removal of the copper the solution is neutralized with ammonia, and excess of the same reagent is then added. The nickel is then precipitated on a clean kathode. Current density ND100 = 0.5 to 1.5 amperes (Classen) and electrode tension 2.8 to 3.3 volts.
This method may be used for copper ores containing nickel (see the article on the “ Determination of Copper by Electrolysis,” by Francis L. Sperry, in Modern Copper Smelting by E. D. Peters, Jnr.).
The Analysis.—Roll out the coin in a pair of rolls or hammer it thin on a clean anvil. Clip it into thin strips with a pair of strong scissors or shears. Weigh out about .5 gm. of the clippings. Transfer them to a deep narrow 250 c.c. beaker. Add 20 c.cs. 5E. HNO3 and warm till the coin is dissolved. Add 8 c.cs. 18E H2SO4 and evaporate on the hot plate till all the nitric acid is removed.
Take up with 120 c.cs. distilled water and electrolyse as before, the most, suitable conditions being as follows — Current density, ND100 = 1 ampere, and the electrode tension 2.75 to 3 volts.
When the copper is all deposited, remove, wash, dry and weigh the kathode. On removing the kathode it should be quickly washed into the beaker containing the solution, by means of a jet of water from the wash bottle. Note the weight of copper deposited.
Clean the kathode. Replace it. Neutralise the solution with ammonia and add an excess of 10 c.cs. 20E. NH4HO.
Pass the current till the nickel is all deposited. The end of the reaction is ascertained by testing with ammonium sulphide or potassium sulpho- carbonate. The coating on the kathode should be thick and bright.
Remove, wash, dry and weigh the kathode, and note the weight of the deposited nickel.
Concluding Note.—The examples given will be sufficient to give the student some practice in this branch of analysis. Further examples will be met with under “ Mixed Analysis.” (See “ Smaltine for Nickel and Cobalt.”).
