Table of Contents
A high-temperature, two-phase extraction technique has been developed for the recovery of tungstic oxide (WO3) from tungsten-bearing minerals. The technique consists of dissolving scheelite (CaWO4) or wolframite in a system of two immiscible liquids-sodium chloride and sodium silicate-at 1,080° C. Tungstic oxide is converted to sodium tungstate (Na2WO4 ) and is transferred to the upper halide phase, and calcium or iron oxide and manganese oxide are retained in the lower silicate phase. The two liquids are then separated by decantation. The halide-tungstate phase containing approximately 28 weight-percent WO3 would be a suitable starting material for the electrolytic recovery of tungsten or tungsten carbide.
A recent Bureau of Mines research program was concerned with developing electrolytic methods for the recovery of refractory metals or compounds directly from mineral concentrates. The electrolytic method for recovery of tungsten or tungsten carbide from wolframite would eliminate several steps from the conventional procedures for preparing these products. The conventional procedures include ore decomposition, preparation and purification of intermediate tungstates or tungstic acid, reduction of oxide to metal, and carburization of metal to carbide.
Although scheelite ore reserves are a more important source of tungsten in the United States, wolframite ores are more abundant in the world. The wolframite isomorphic series, from ferberite (FeWO4) to huebnerite (MnWO4), is an important domestic source of tungsten. Large deposits occur in Vance County, N.C.; Lemhi County, Idaho; and Boulder County, Colo. Also, large quantities of wolframite concentrate byproducts are recovered at the Climax Molybdenum Mine in Colorado.
Electrowinning of tungsten from wolframite dissolved in sodium phosphate or sodium tetraborate electrolytes was investigated by Fink and Ma. These investigators did not report the analysis of iron, manganese, and other impurities in their products. Commenting on the preparation of tungsten metal by electrolysis of its minerals, Andrieux stated that direct electrowinning of high-purity tungsten from the minerals did not appear practical because of the codeposition of iron and other impurities. The Bureau of Mines had previously developed electrolytic techniques for winning tungsten metal from tungstic oxide and scheelite, and for preparing tungsten carbide from sodium tungstate.
The objective of the present study was to develop electrolytic methods for the recovery of tungsten from wolframite. Two methods to recover tungsten as metal and as carbide were investigated: (1) Directly from wolframite and (2) from the halide-tungstate melt obtained from two-phase extraction of wolframite. The results of these two methods are compared,
Materials Equipment and Procedure
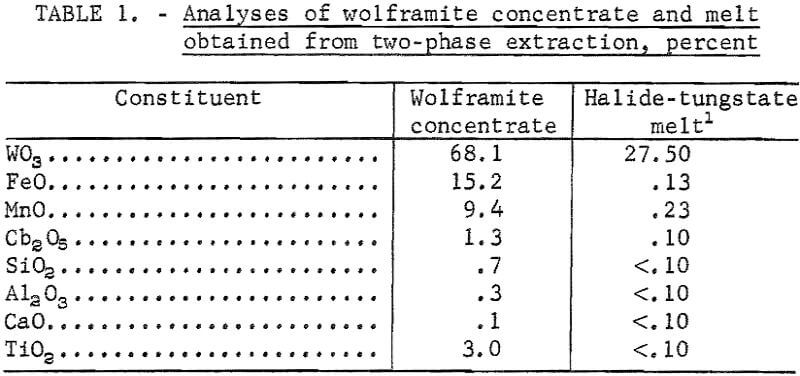
Wolframite ore concentrate from Colorado was used in the direct electrowinning experiments and in the two-phase extraction from which a halide-tungstate melt was obtained for subsequent electrolysis. The analyses of the wolframite concentrate and the halide-tungstate melt are shown in table 1.
The procedure for making the two-phase extraction was described in a previous publication. Three parts of wolframite concentrate, 5 parts of sodium chloride, and 2 parts of sodium metasilicate (Na2SiO3) were mixed, charged in a silicon carbide crucible, and heated to 1,080° C. After holding the mixture at this temperature for 1 hour two immiscible liquids were formed. The upper halide-tungstate melt was very fluid and easily decanted from the lower, viscous silicate melt.
The electrolytic cell consisted of a graphite crucible, 3 inches in inside diameter and 7 inches deep, which served as the anode. The cathode was a 1-inch-diameter graphite rod, centrally positioned in the cell. The cell was heated in an electric furnace.
In the electrowinning of tungsten directly from wolframite, approximately 1,000 grams of a mixture of wolframite concentrate and various phosphate, borate, and halide reagents were used in making up the electrolyte. The mixture was heated to 1,000° C. The electrolysis was conducted at the following conditions: Current, 90 amperes; initial cathode current density, 150 amp/dm²; and cell voltage, from 2.5 to 5.2 v. After 60 amp-hr of electrolysis, the cathode was withdrawn from the electrolyte, and the deposit was scraped off the hot cathode.
In electrowinning from the halide-tungstate melt, the electrolyte was composed of 800 grams of the melt and approximately 200 grams of phosphate and borate reagents. The electrolyte was heated to 1,000° C. In the electrodeposition of tungsten metal, the operating conditions were as follows: Current, 90 amperes; initial cathode current density, 150 amp/dm² ; cell voltage, from 2.3 to 2.6 v; and 60 amp-hr of electrolysis. In the electrodeposition of tungsten carbide, the electrolyte was composed of the halide-tungstate melt with additions of sodium chloride, sodium metaborate, and sodium hydroxide. The current and initial cathode current density were the same as stated above, but the cell voltage was from 2.8 to 3.5 v, and the electrolysis was conducted for 90 amp-hr. After electrolysis, the cathode was withdrawn from the electrolyte, and the deposit was scraped off the hot cathode.
The deposits were first leached in a hot 5-percent HCl solution, followed by leaching in a 2-percent NaOH solution to remove the entrained electrolyte. The products were then washed with water, dried, weighed, and analyzed. The metallic impurities were analyzed by a spectrographic method, and total carbon was analyzed by a combustion-gas chromatographic procedure. In a few instances, free carbon was also analyzed, using a chemical separation and the combustion-gas chromatographic procedure.
Experimental Results
Direct Electrowinning of Tungsten Metal From Wolframite
Electrowinning of tungsten directly from wolframite concentrate was studied with electrolytes consisting essentially of various phosphate, sodium borate, and alkali halide reagents. Some combinations of these compounds were successfully used as electrolytes for electrowinning tungsten from wolframite by Fink and Ma and from scheelite and tungsten oxide by the Bureau of Mines. The compositions of the electrolytes and the results of the present study on electrowinning tungsten are shown in table 2.
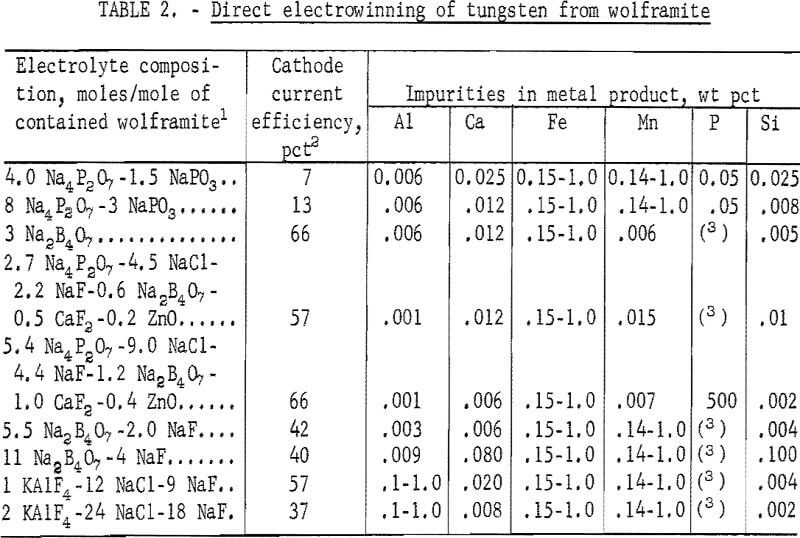
Deposits won from each of the electrolytes investigated contained more than 0.15 percent iron. The manganese content was over 0.14 percent in the deposits produced from the following electrolyte systems: Na4P2O3-NaPO3, Na2B4O7-NaF, and KAlF4-NaCl-NaF. The aluminum content was in excess of 0.10 percent in the products obtained from electrolytes containing potassium aluminum tetrafluoride (KAlF4). The overall results supported Andrieux’s statement that direct electrowinning of tungsten from minerals would incur contamination from the associated mineral constituents. Preparation of high-purity tungsten metal directly from wolframite by electrolysis does not appear promising because of the codeposition of impurities.
Electrowinning of Tungsten Metal From Wolframite Via Two-Phase Extraction
Deposits were not produced by the electrolysis of the halide-tungstate melt as obtained from the high-temperature extraction treatment of wolframite. Guided by previous work on electrowinning of tungsten, investigations were made with electrolytes prepared by additions of phosphates, borates, and/or alkali aluminum fluoride to the halide-tungstate melt. Tungsten deposits in various states of purity were obtained in these electrolytes. Products with over 0.15 percent iron and 1.0 percent carbon were obtained from electrolytes containing sodium aluminum fluoride (Na3AlF6 ) or potassium aluminum fluoride (KAlF4). Products with up to 0.15 percent iron, 0.14 percent manganese, and 0.06 percent silicon were obtained from electrolytes containing alkali metaphosphate or pyrophosphate. Products with 0.30-0.1 percent iron and 0.03-0.13 percent carbon were obtained from electrolytes containing boric oxide or sodium tetraborate (Na2B4O7). The highest purity tungsten metal was obtained from electrolytes containing both NaPO3 and B2O3 ; therefore, these electrolytes were studied further. Results from electrolytes containing various amounts of these two compounds are shown in table 3.
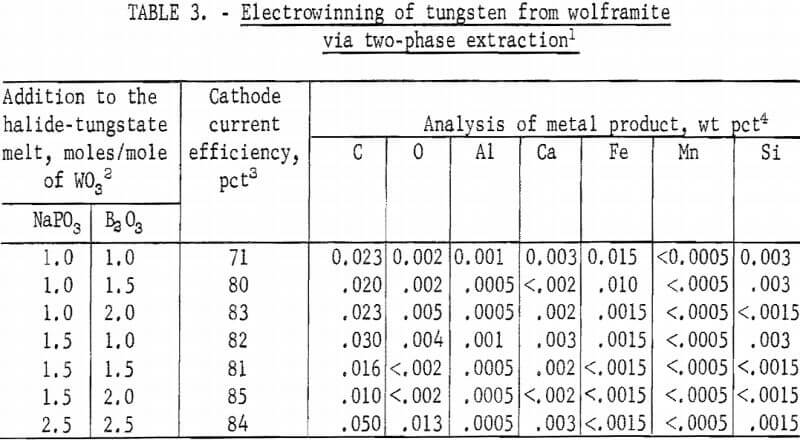
The quality of the product did not change greatly with varying proportions of NaP03 and B2O3 in the electrolyte composition. With the exception of the electrolyte containing 1.0 mole of NaPO3, and 1.0 mole of B2O3 per mole of WO3, the cathode current efficiencies for all the electrolyte compositions were surprisingly uniform, within a narrow range of 80 to 85 percent. Best cathode current efficiency and product quality were obtained with the electrolyte composition containing 1.5 moles of NaPO3 and 2.0 moles of B2O3 per mole of WO3.
Electrodeposition of WC from Wolframite
Attempts to electrodeposit WC directly from wolframite were not successful in producing the material in an acceptable state of purity. Products obtained in the experiments were mixtures of WC, ditungsten carbide (W2C), tungsten metal, and free carbon. Moreover, the products also contained iron and manganese impurities, each in the range of 1 to 5 percent. Because of the impurities in the products, electrodeposition of WC directly from wolframite was not pursued. Efforts were subsequently directed toward using the halide-tungstate melt obtained from the two-phase extraction.
In previous work on electrodeposition of WC from Na2WO4, the optimum electrolyte composition was determined to be 83 mole-percent NaCl and 5.7 mole-percent each of Na2WO4, Na2B2O4, and NaOH. In experiments to deposit WC from the halide-tungstate melt, 1.0 mole each of Na2B2O4 and NaOH was added per mole of WO3 in the melt. The quantity of NaCl in the electrolytes was varied from 75 mole-percent, the quantity in the original halide-tungstate melt, to 86 mole-percent, an addition of 9.0 moles of NaCl per mole of WO3. The results are shown in table 4.
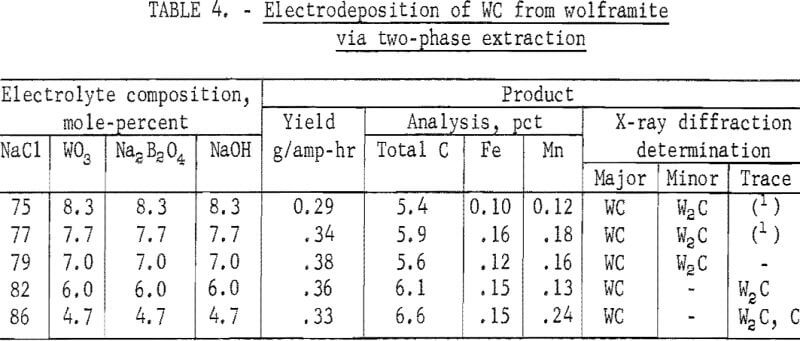
The results in table 4 indicate that a greater proportion of W2C was formed from electrolytes containing 79 mole-percent or less of NaCl. The presence of W2C in these products was indicated by X-ray diffraction as well as by the analytical results for carbon which showed values below 6.1 percent, the stoichiometric carbon content in WC. On the other hand, free carbon was detected in the product obtained from the electrolyte containing 86 mole-percent NaCl.
Over 90 percent of the tungsten value in the electrolyte containing 82 mole-percent NaCl was recovered by electrodeposition. X-ray diffraction and the total carbon analysis showed that the product was nearly all WC. A trace quantity of W2C in the material was indicated by X-ray diffraction, and the presence of 0.07 percent free carbon was determined by analysis (not shown in table 4). Spectrographs analysis of the material also indicated the following impurities present in weight-percent: Al, <0.001; B, 0.0015; Ca, <0.0015; Cr, 0.003; Cu, 0.02; Mg, 0.004; Mo, 0.02; Ni, 0.001; Si, 0,008; Sn, 0.01; Ti, 0.001; and V, 0.003. The microhardness of this product, determined with a 50-gram load, was 1,700 Vhn.
Conclusions
Tungsten metal of 99.9 percent purity was electrowon from a halide-tungstate melt that was obtained from a high-temperature, two-phase extraction treatment of wolframite. This high-purity product was electrodeposited when sodium metaphosphate and boric oxide were added to the melt to form an electrolyte. Other tungsten metal produced by direct electrolysis of wolframite dissolved in molten salt mixtures composed essentially of various combinations of sodium phosphates, sodium borates, and sodium halides was of lower purity, containing about 0.15 percent each of iron and manganese.
Tungsten carbide was also recovered from the halide-tungstate melt. The electrolysis was performed in an electrolyte composed of the halide-tungstate melt and additions of sodium metaborate, sodium chloride, and sodium hydroxide.
