For characterising the electrical behaviour of chemically conditioned mineral samples and after charging the minerals with the body of the stainless steel mill they are subjected to an electrostatic free fall separator by using a steel feeder.
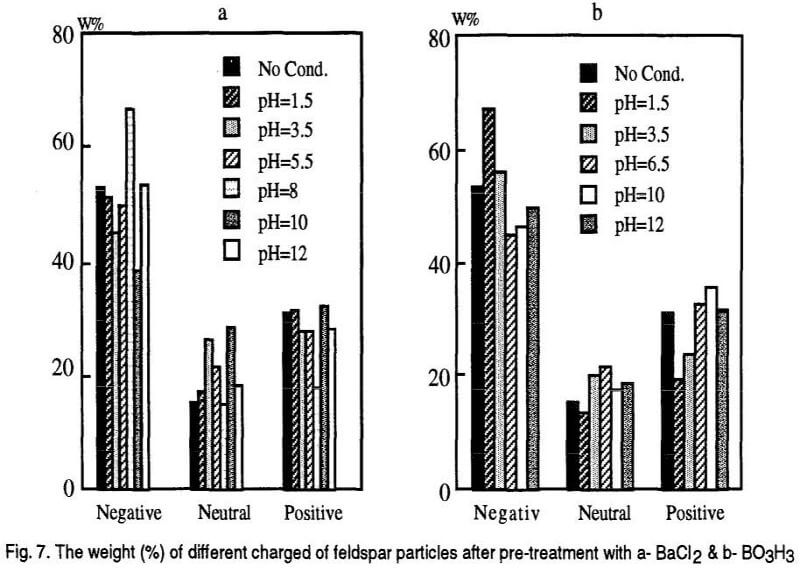
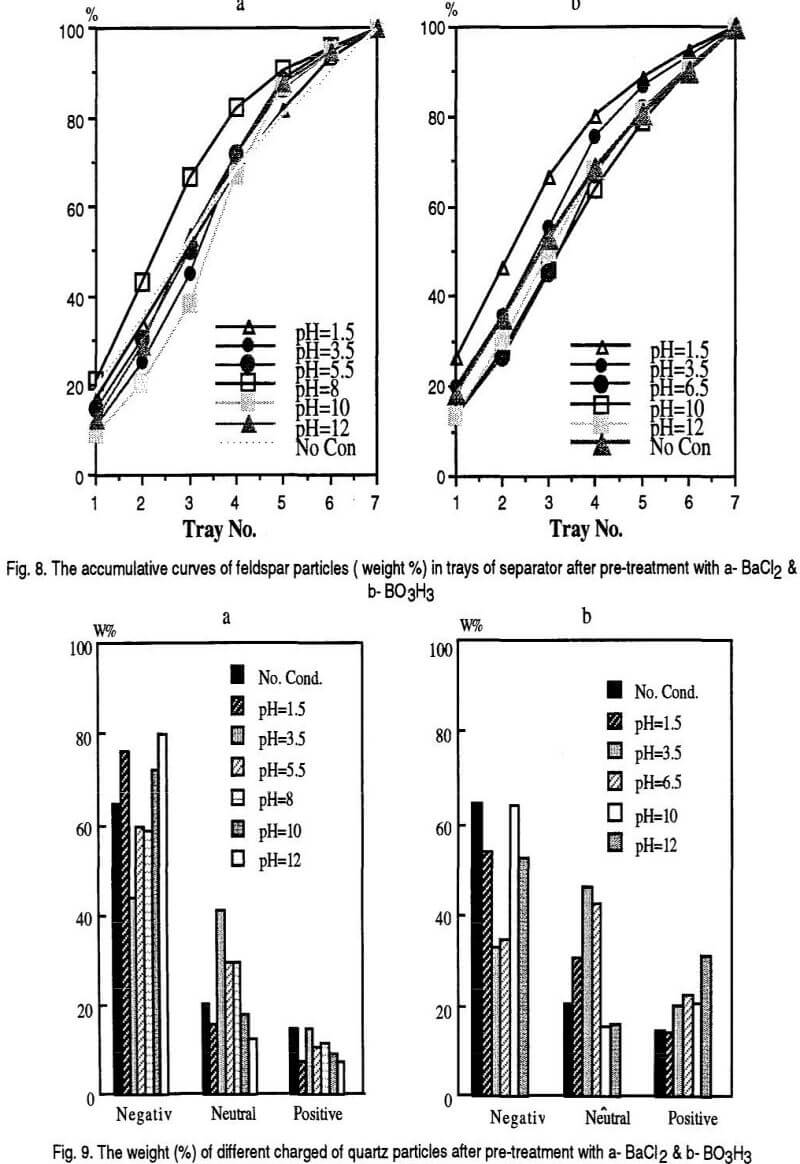
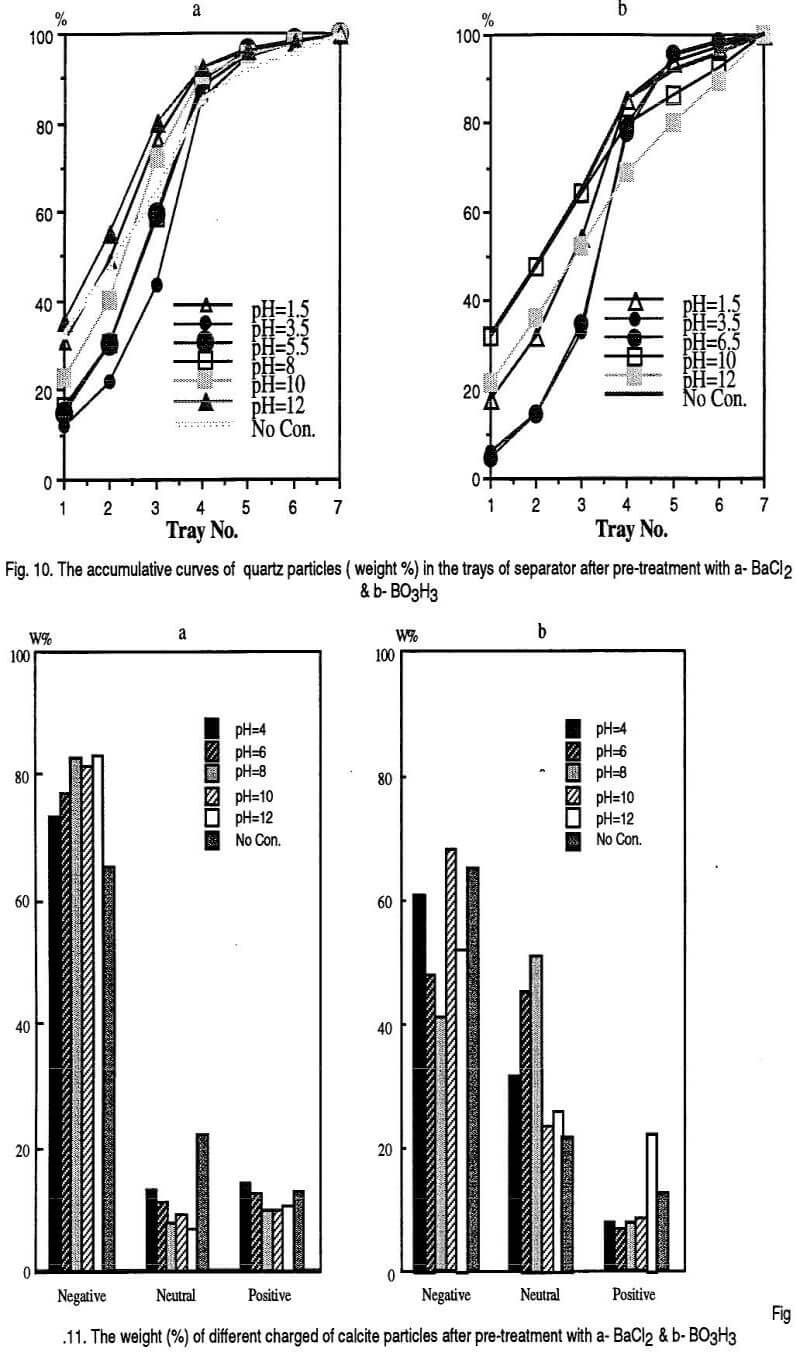
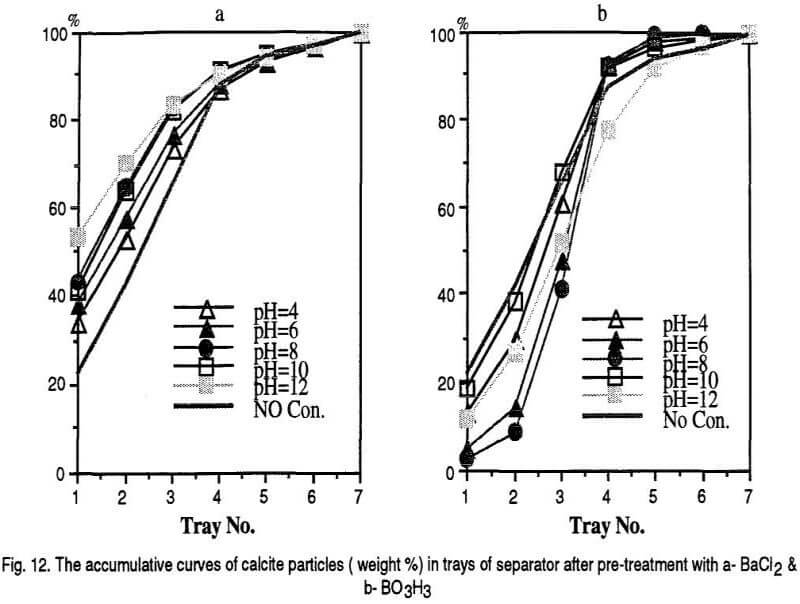
Based on the polarity and the magnitude of the charges acquired by mineral grains they are attracted by the positive and negative electrodes and different trays of the separator are occupied. It is assumed that the first three bins of the separator are the places for negatively charged particles, the last three bins are for the positively charged particles and the middle bin is the place for neutral particles. The results are shown in the following Figs. 7, 9 and 11 however, The accumulative curves are shown as the Figs. 8, 10, and 12.
The separation process results for quartz mineral are generally in accordance with the theory. A higher concentration of cation species are formed by dissociation of the BaCl2 at pH<3 and pH>11 values which result accumulation of the quartz particles in the first three bins of the separator. The accumulation curves of quartz samples treated with H3BO3 are less attracted by positive electrode compared to non-conditioned sample. It can be observed that, for both chemicals at pH 3.5 value, less quartz particles occupy the first three bins of the separator.
Generally, calcite particles are attracted by the positive electrode when BaCl2 is used as the conditioning reagent. However, the middle bin of the separator is occupied by more calcite particles in the case of H3BO3 pre-treatment. These can be explained by adsorption of the barium cations and borate anions on calcite particles respectively. It can be observed that the accumulative curves for calcite mineral after BaCl2 and H3BO3 pre-conditioning shift above and below the non-conditioned sample respectively.
Interpretation of the response of feldspar to the electrical field seems to be difficult and far from theoretical basis mentioned before. Although, the adsorption of cations and anions on feldspar mineral seems to be in accordance with its surface charge but, its electrical separation response is not as expected.
Although remarkable results are found by charge measurements, no distinguished changes in electrical beneficiation potential of primary charged minerals with copper and PVC plates compared to steel are seen. The convincing reason is the use of steel feeder for all separation tests. It means that ultimately, a new charge is developed on each sample after contacting with the feeder plate that is mainly influenced by the energetic levels of steel plate.
It must be added that during the separation the bins nearer to negative electrode are occupied by finer mineral grains. The coarser particles were collected by the positive electrode.
This observation can be explained by the classical electrostatic description of the work function and ionization energy of the insulators (Gallo & Lama, 1976). The energy required to extract an ion is inversely related to the size or thickness which means that the work function is smaller for larger particles of the same material. Consequently, there is a natural tendency to transfer electrons (i.e., charges) from larger particles to smaller particles. Therefore, finer particles in contact with coarser ones can acquire a negative charge then after contacting with the feeder they are tending to hive off the electrons and finally the negative electrode attracts them
In spite of the existence of different effective factors on contact electrification phenomena, it seems to be possible to modify the magnitude and the sign of charge on mineral in a particular separation process by chemical treatment of material surfaces. What could happen on mineral surfaces after chemical treatment can be explained by adsorption of the either anions or cations. Theoretically, a mineral acquiring negative charge must possess a thermionic work function greater than that of the charging surface. This work function should be reduced by adsorption of negative ions onto the mineral surface (Pearse & Pope, 1977). On the contrary, adsorption of the positive ions causes an increase in the work function because of introduction of holes on mineral surfaces. Therefore it must be possible to either increase or decrease the magnitude of the triboelectric charging or even change the sign of the charge by chemical preconditioning.
The reaction at the surface of a semi-conductor may involve either decomposition of the semi-conductor itself, or exchange of charge with an ion in the electrolyte. When decomposition occurs, a new surface with its own electrical behaviour appears and changes both the surface and bulk electrical properties of the primary mineral. However, in the case of exchanging the charge carriers the surface adsorbs either anions or cations or both. Consequently, the electrical properties (e.g., conductivity) of the specific mineral is affected by setting a new balance between the charge carriers of mineral and the adsorbed chemical(s).
Not only the tendency of the chemicals, solution pH and concentration but also the charge type of the minerals (i.e., p or n type semi-conductor) determine the adsorption process. If there is a chemical reaction between the minerals and solution, a new surface with new electrophysical properties and new electrical separation behaviour appears. The adsorption of ions on the surface of the minerals according to their electrical behaviour and surface charges as well as concentration and donicity of the chemicals results new surface with new energetic levels.
The results showed that the main electrical properties, the magnitude and polarity of the accumulated charges on mineral grains can be artificially modified by chemical pre-treatment contributing to the electrical beneficiation potential of minerals. During electrical separation of non-conditioned quartz, calcite and feldspar, more than 50% of these mineral grains are attracted by the positive electrode. However, after pre-treatment their electrical responses have changed.
