Table of Contents
The phosphate ores mined in middle Tennessee typically consist of granular rock phosphate particles disseminated in a clayey matrix. In the TVA plant near Columbia, Tenn., the phosphate ore is mined, made into a slurry with the addition of a small amount of sodium hydroxide as dispersant, and treated in a hydroseparator to remove minus 10 micron material. The hydroseparator underflow, comprising a rough concentrate, is transported by pipeline to the plant; the hydroseparator overflow, comprising a tailing, is flocculated by addition of calcium sulphate and is discharged to settling ponds at a rate of about 1400 gpm. Sedimentation in the ponds produces a clarified effluent, which may be recycled for use as process water or discharged to surface drainage. This method of tailing treatment is not entirely satisfactory since poor sedimentation characteristics of the tailing result in poor ultimate utilization of pond storage volume. The sediment contains about 70 pct water, even after several years of settling, and is not sufficiently dry to permit it to be used as back-fill, which would provide a method for reclaiming ponds that had been filled with sediment.
Initial Considerations
Electrophoresis may be defined as the migration of particles in liquid suspension resulting from the application of direct current electricity to the suspension. This phenomenon has been found to be applicable to the separation of particles from suspension by deposition on an electrode immersed in the suspension. Although the principles of electrophoresis have been the subject of numerous laboratory investigations, there have been few commercial applications of the phenomenon to the separation of solids from liquids. This, according to Creighton, is probably due in part to lack of knowledge of conditions necessary for best results. It is also due to the availability of simpler and better-known methods that will usually accomplish solids separation at lower cost, namely, settling, filtering, or centrifuging.
In work on the electrical dewatering of clays, Speil and Thompson found the following conditions to give the best results: high solids concentration in slurry, low conductivity of slurry, high temperature, close spacing of electrodes, and effective mechanical circulation to bring the suspended particles close to the anode. The kind and dosage of dispersant chemical were shown to have important effects that depended on the particular clay being treated. In the present study, an effort was made to fix these variables as near to expected optima as possible prior to starting experimental work.
Electrical Dewatering Tests
Equipment design, materials, and procedures used in the experimental work were selected with a view to applicability to plant-scale operations. Of the several types of electrophoresis machines proposed in the literature, two appeared to be promising for this specific application. These were (1) the rotating drum type and (2) the continuous-belt type. The rotating drum machine is similar in appearance to a rotary vacuum filter except that the drum has a solid conducting surface that serves as the anode; the solids adhere to this drum and are scraped off with a fixed blade. The continuous-belt type machine has for an anode either an endless metallic strip partly immersed in the slurry or a stationary metal plate over which is drawn an endless cloth strip.
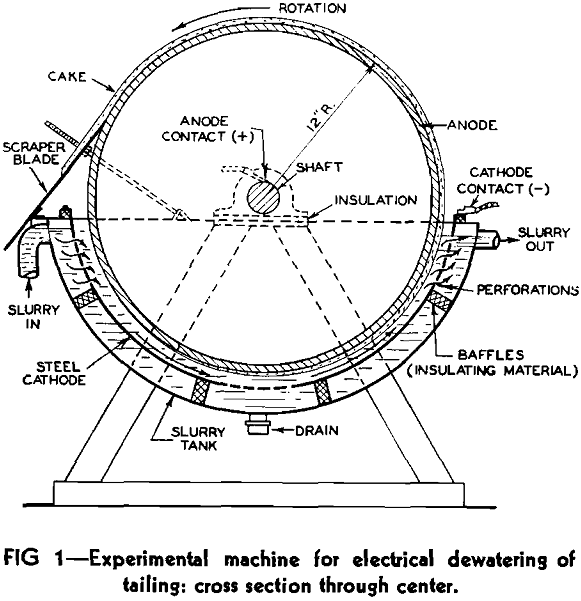
An experimental electrophoresis machine for the dewatering tests was constructed based on Schwerin’s idea a cross-sectional sketch of the machine is shown in Fig 1. The principal parts were: a semicylindrical tank to hold the slurry, a semicylindrical sheet-steel cathode suspended in the tank, and, concentric with this, a revolving drum of electrically conductive material that dipped into the liquid and served as the anode. A fixed scraper blade was provided to remove deposited solids from the surface of the rotating drum; this was set so that it would just clear the drum surfaces without effecting metal-to-metal contact.
Since the experimental work was not carried out at the Columbia, Tenn., plant, but at the Wilson Dam, Ala., laboratory of TVA, it was not convenient to use tailing slurry as produced in the hydroseparator. Test slurries for experimental work were prepared in the laboratory from tailing sediment dug from a settling pond at the plant and shipped in moist condition to Wilson Dam. Although the composition of the tailing varied somewhat, the following analysis represents a typical sample in per cent by weight on dry basis: P2O5, 9.0; CaO, 10.0; SiO2, 37.4; Fe2O3, 7.9; Al2O3,18.2; loss on ignition, 11.5.
Effect of Deposition Time
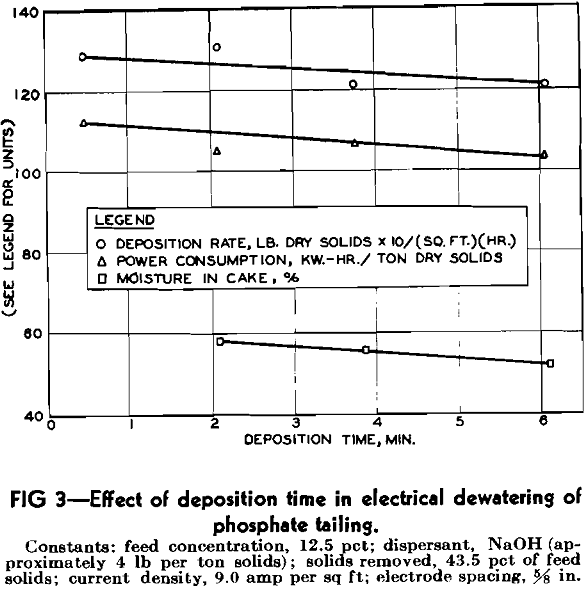
In the electrical dewatering of clays, Speil and Thompson found that the optimum deposition time was that which allowed sufficient dewatering of the deposit without increasing the resistance of the deposit to such a point that the energy consumption per unit of clay increased noticeably. The optimum deposition time varied with different clays. In the present study a series of tests were run in the experimental dewatering machine to determine the effect of deposition time. For these tests, an electrode spacing of 5/8 in. was used so that deposition time, hence cake thickness, could be varied over a wider range than would be permitted by the 3/8 in. spacing that was considered to be the minimum practical for large-scale use. Although the wider spacing caused high power consumptions, the tests served to indicate the relative effects of deposition time on power consumption and on other process variables. The current density was held at 9 amperes per square foot; feed slurry concentration was 12.5 pct; the duration of each test was 30 min; and sodium hydroxide was used as the dispersant at a dosage of about 4 lb per ton of solids, which is the dosage generally used in the plant.
Effect of Dispersant Chemical
With regard to the best kind and amount of dispersant chemical, preliminary tests showed that sodium hydroxide in a dosage of about 4 lb per ton of tailing solids or, as an alternative, sodium metasilicate at 5.2 lb per ton would be equally suitable from the standpoint of obtaining satisfactory dispersion of the phosphate pulp. Laboratory tests with several other chemicals showed that, although satisfactory dispersion could be obtained with some, the costs for the required dosages would be considerably greater than those for sodium hydroxide or sodium metasilicate. The study of effect of dispersant on electrical dewatering was confined, therefore, to a consideration of these two chemicals, and conductometric titrations were made to determine their relative effects on slurry conductivity since electrophoretic efficiency in dispersed slurries was known to be inversely proportional to conductivity.

