The effects of oxidation on the flotation behavior of sulphide minerals have been extensively studied, but no similar study has been made of coals. Coals of bituminous and lower rank undergo atmospheric oxidation during mining and storage, and their degree of oxidizing increases with temperature and time of exposure. The problem of recovering fine oxidized coal from culm piles, rivers, and streams by froth flotation has become increasingly important to the coal industry as well as to those concerned with air and stream pollution.
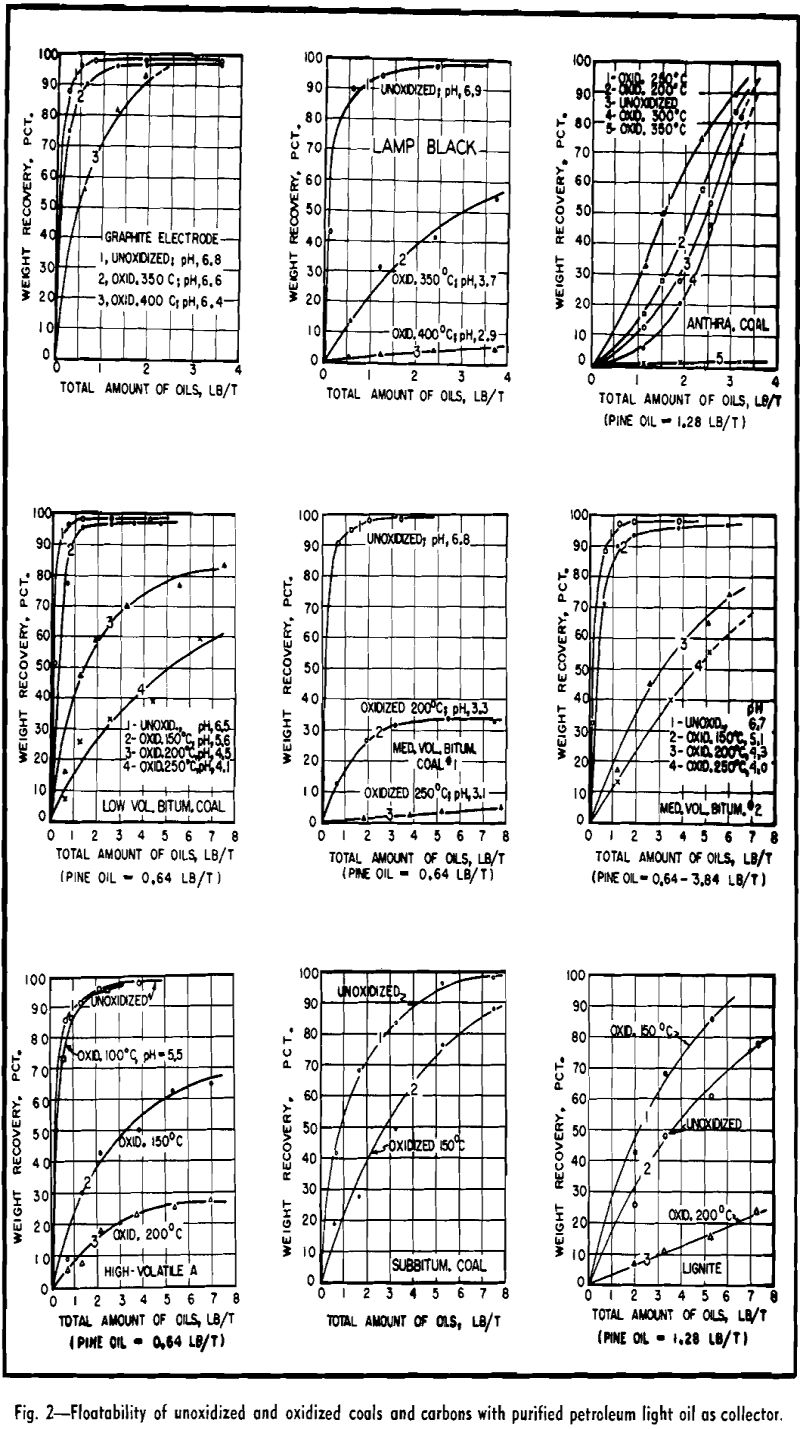
Flotation tests of unoxidized and oxidized coals and carbons were performed with purified petroleum light oil as collector. Similar results, not presented, were also obtained by parallel tests with other oily collectors such as kerosene, decane, dodecane, and tetradecane.
Upon review of the analytical data, the above flotation phenomena can be explained by the surface components hypothesis. For example, considering point 1, the high floatability of bituminous coal is attributed to its high contents of floatable components including hydrogen and carbon, in conjunction with its relatively low percentages of non-floatable components including water, oxygen, and ash. The comparatively low floatability of subbituminous coal is caused by a relatively high content of water and oxygen as well as a slight decrease in hydrogen and carbon contents. The low percentage of hydrogen is considered the main reason for the
still lower floatability of anthracite coal, whereas the extremely high percentages of water and oxygen cause the very low floatability of lignite.

The chemical properties of the oxidation products of coals have been studied by many investigators. It is generally accepted that the oxidation of coals can be divided into three stages. Stage 1 is a superficial oxidation characterized by the formation of coal-oxygen complexes with acidic properties. In stage 2, a large portion of the organic components of coal, including hydrocarbons and carbon, is transformed into hydroxycarboxylic acids, generally called humic acids. In stage 3, the humic acids are further degradated to water-soluble acids. Under the same oxidation conditions, the ratio between the benzoid and oxalic acids in the oxidation products of coals increases with the maturity of the coal.
An attempt was made to determine whether the low floatability of a mildly oxidized coal is caused by its water-insoluble oxidized surface or its water- soluble oxidation products in the flotation pulp. The test results show that the water-insoluble surface of the oxidized coal is chiefly blamed for its low floatability. This is due to the fact that the surfaces of considerably oxidized coals, predominated by hydroxyl and carboxyl groups, are water-avid and oil-repellent.
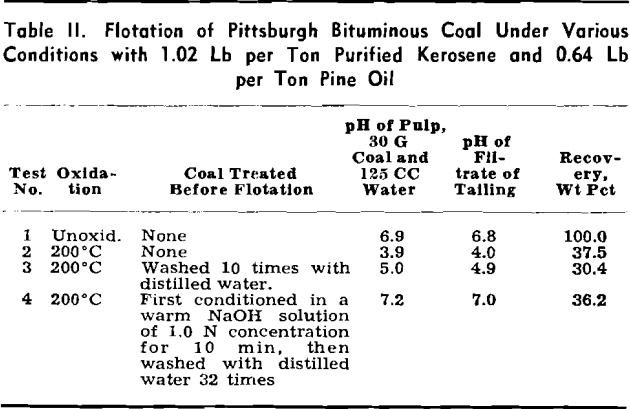
The influence of water-soluble oxidation products and some of their constituents on the floatability of Pittsburgh bituminous coal was ascertained by means of flotation, quantitative bubble pick-up, and frothing tests. The experimental data indicate that, in the flotation of unoxidized coals, the behavior of water-soluble products resulting from various stages of oxidation of coals is generally as follows: A—stage 1, weak collectors; B—stage 2, mixtures of weak collectors and weak depressants; and C—stage 3, mixtures of weak and moderate depressants. The same data indicate also that the water-soluble products resulting from all the stages of oxidation of coals are weak frothers. As a whole, the effect of the water- soluble products in lowering floatability is relatively unimportant, as compared with the important role played by the water-insoluble oxygenated surfaces of coals.