Table of Contents
Critical pH has been defined by Wark as that pH value below which a mineral will float and above which it will not float in solutions containing a given concentration of collector but free from other depressants or activators. The relationship between mineral, collector concentration, and pH is expressed in the form of a critical pH curve determined by the captive bubble method and is generally regarded as being fundamental to the system involved. The mechanism of alkali depression is currently in dispute, two general theories having received authoritative support.
Alkali Depression of Cerussite by Control of Carbonate Ion Concentration
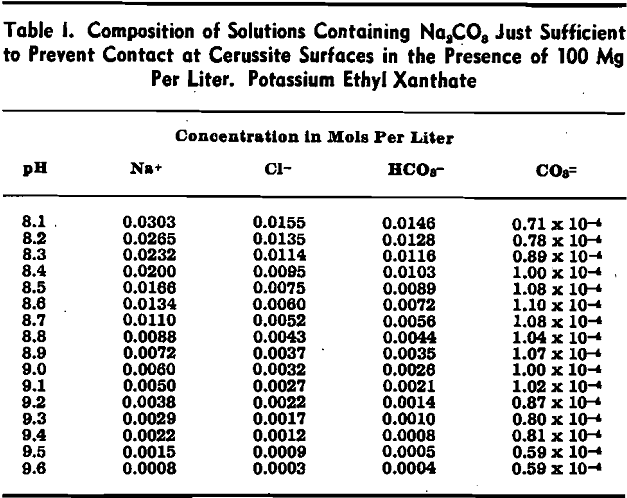
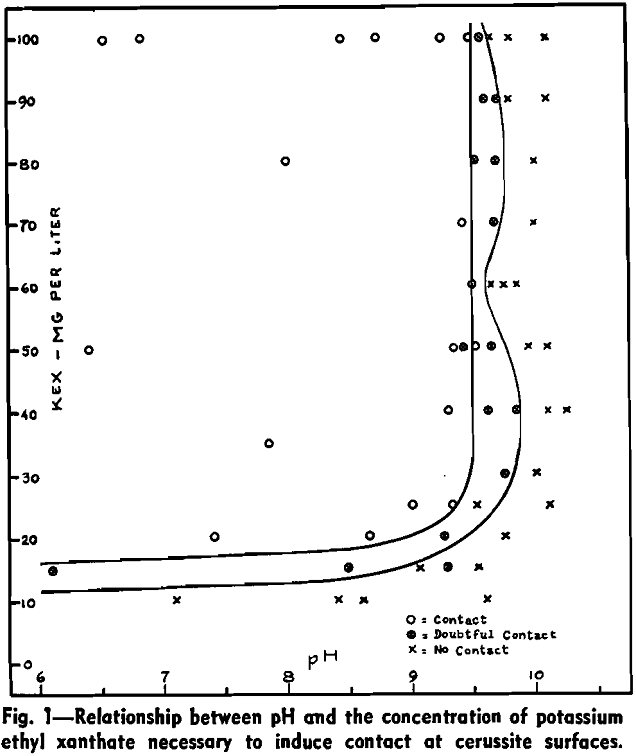
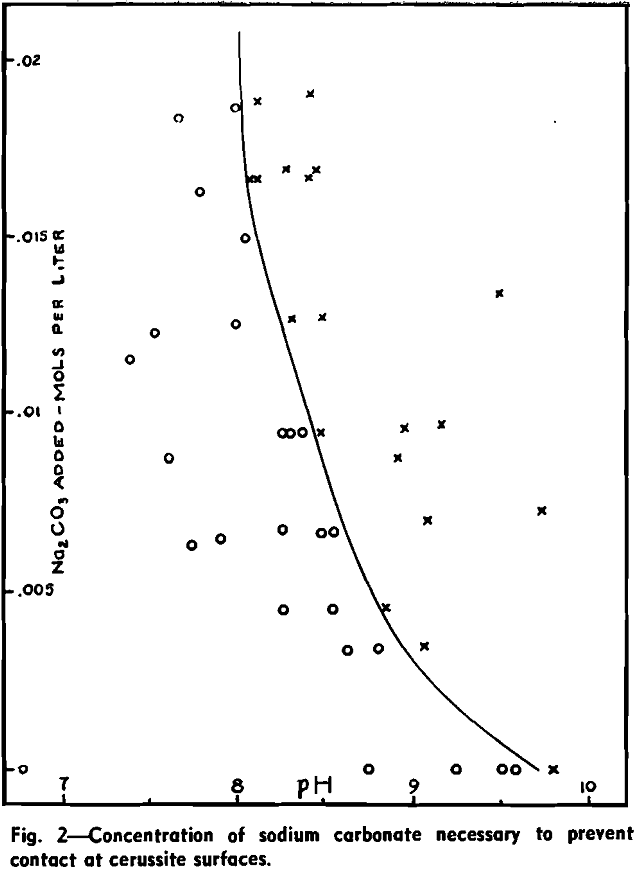
Wark and Cox have shown that in the presence of Na2CO3 a higher concentration of xanthate is required to effect bubble contact with a cerussite surface than in carbonate-free solutions. To examine this depression of cerussite by sodium carbonate, a series of captive bubble tests was carried out on cerussite specimens in solutions containing 100 mg per liter of potassium ethyl xanthate and varying concentrations of sodium carbonate. The pH was adjusted by the addition of dilute hydrochloric acid.
The carbonate solutions contained Na+, K+, Pb++, OH-, Cl-, CO3=, HCO3- and EX- and, at the concentrations used, dissociation may be assumed to be complete. Na, K, H, Cl, and EX were measured quantities. By introducing these known values into the statement of electrical neutrality for the system and combining this with the second dissociation constant of carbonic acid and the ionic product of water, it is possible, with a little algebraic manipulation, to calculate the missing factors.
The critical carbonate ion concentration for air-cerussite contact in the presence of 100 mg per liter of potassium ethyl xanthate may, then, be taken as 1.0×10 -4 mols per liter at 25°C.
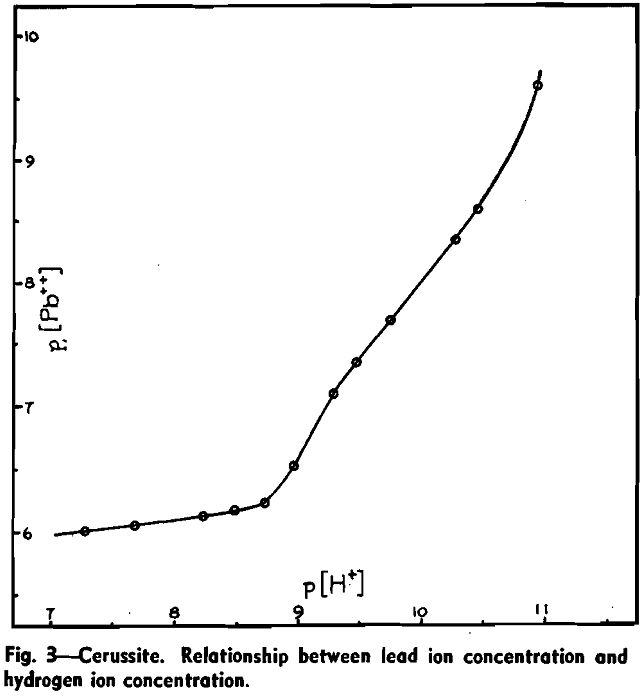
To study the reaction between cerussite and alkali a galvanic cell capable of registering small changes of lead ion concentration was employed:
Pb | Pb++ (aq) : KCl (aq saturated), HgCl2 | Hg.
The lead electrode was specially purified rod, 99.9988 pct Pb, supplied by Johnson Matthey & Co. The test solution vessel contained a mechanical stirrer and a glass electrode which, by a special switching arrangement, could replace the lead electrode in the circuit so that EMF and pH could be measured alternately. The complete cell was suspended in a thermostatic bath at 25 °C.
3Pb CO3 + 2 OH- = Pb3 (CO3)2(OH)2 + CO3=
In alkaline solutions, basic lead carbonate does not dissociate to yield free lead ions but, as Randall and Spencer have shown, reacts with more hydroxide in the following manner:
Pb8(CO3)2 (OH)2(solid) + 7 OH- = 3HPbO2- + 2CO3= + 3H2O
The presence of excess CO3= over and above that produced by the dissociation would repress these reactions and, indeed, in carbonate solutions at low alkalinity, basic lead carbonate is dissociated with the formation of Pb++ and HCO3- :
Pb3 (CO8)2(OH)2 (solid) + 4H2 CO3 (aq) = 3Pb++ + 6HCO8- + 2H2O
Alkali Decomposition of Lead Xanthate
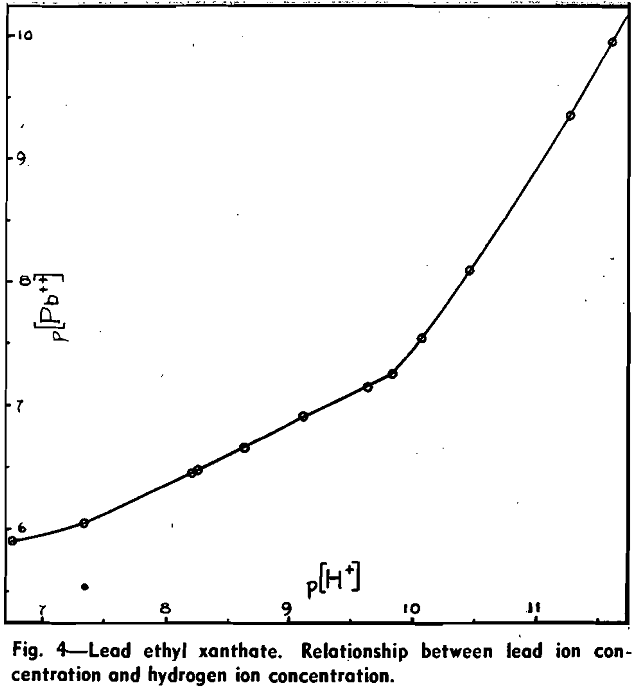
Using the galvanic cell and experimental procedure previously described, the influence of increasing concentrations of potassium hydroxide upon the lead ion concentration of aqueous solutions in contact with solid lead ethyl xanthate was measured.
During these experiments it was observed that upon the addition of KOH which raised the pH from 9.82 to 10.07, the solution, which had hitherto been colorless, assumed a distinctly yellow shade and the solid lead xanthate became tinged with brown. Further additions of alkali accentuated this change and, at the end of the test, the liquid was distinctly yellow and the solid residue had turned dark brown. This was confirmed by a series of tests in which solid lead ethyl xanthate was placed in solutions of varying alkalinity and changes were observed over a period of 20 hr.

Alkali Decomposition of Collector Coatings at Galena Surfaces
A series of captive bubble tests were carried out in which galena was first conditioned in neutral potassium ethyl xanthate solutions and then transferred to solutions of varying pH. In transferring from one solution to another the specimen was held face down in glass tongs while 50 ml of solution was poured over it as a rinse.
These observations suggest that the collector coated surface of galena is, in effect, lead xanthate and that the depressant action of high pH in flotation is due to the decomposition of that surface as described in this paper under Alkali Decomposition of Lead Xanthate. The relation of items 1 to 5, above, to this hypothesis is self-evident.
The tests described show that no general theory of alkali depression is tenable, for the mechanism of depression depends upon the nature of the mineral-collector system concerned. It has been shown that the action of carbonate-free alkaline solutions in preventing the flotation of cerussite may be independent of the collector and may be due entirely to decomposition of the mineral surface.
