The two size reduction/froth flotation interactions described in this paper are strongly related to optimally using the inherent electrochemical surface rearrangements that occur on selected sulfide minerals, e. g. chalcopyrite, molybdenite, pentlandite, etc. Based on data generated in numerous fundamental studies, it is not surprising that utilizing the electrochemical transformations that occur with grinding could make a difference in downstream froth flotation. What is surprising is the magnitude of the electrochemical effects that can be positively or negatively used in larger scale equipment.
It has been known for some time, that certain sulfide minerals such as chalcopyrite, molybdenite, pentlandite, sphalerite, etc. can demonstrate natural or collectorless flotation under various conditions. In practice, such collectorless flotation is difficult to control and maintain recovery so that some level of chemically assisted hydrophobing action is required. It is also evident from both fundamental and plant studies that standard thiol collectors such as xanthates, dithiophosphates, etc. can and do exhibit some electrochemically controlled character. However, from a practical viewpoint, the adsorption mechanisms involved with such collectors are extremely complex and the specific electrochemical nature of standard thiol collectors is difficult to control in a plant environment.
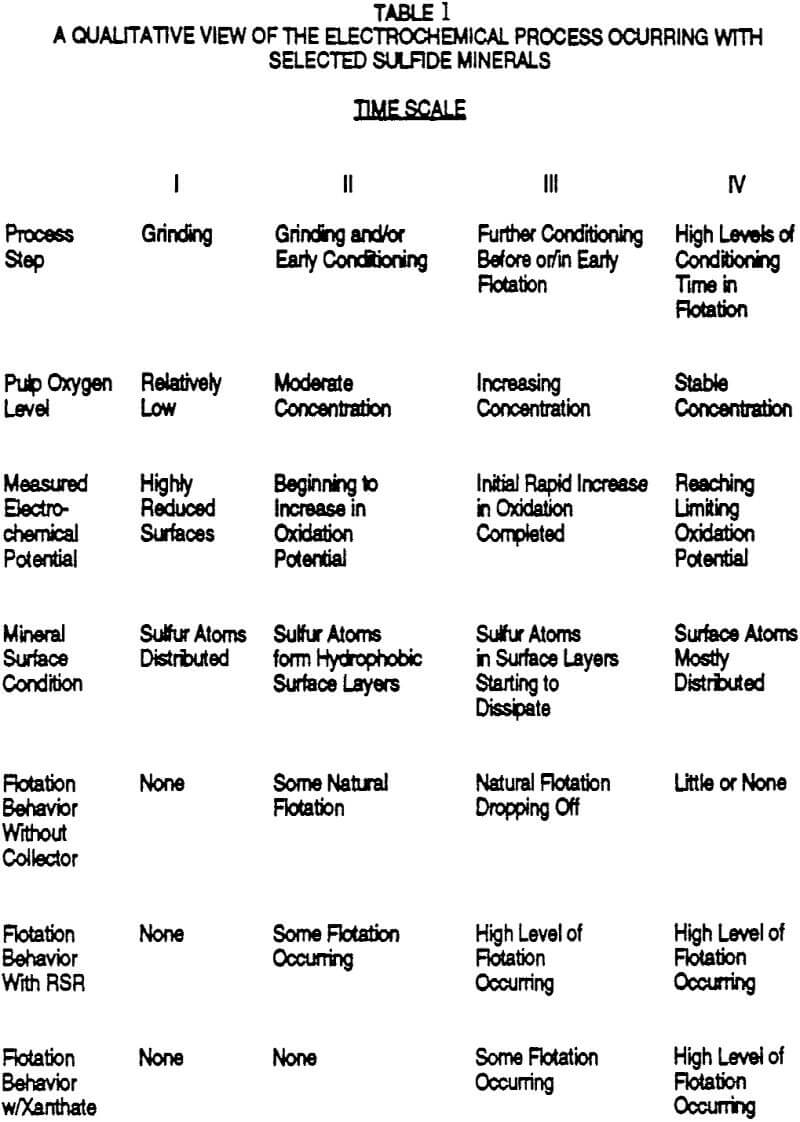
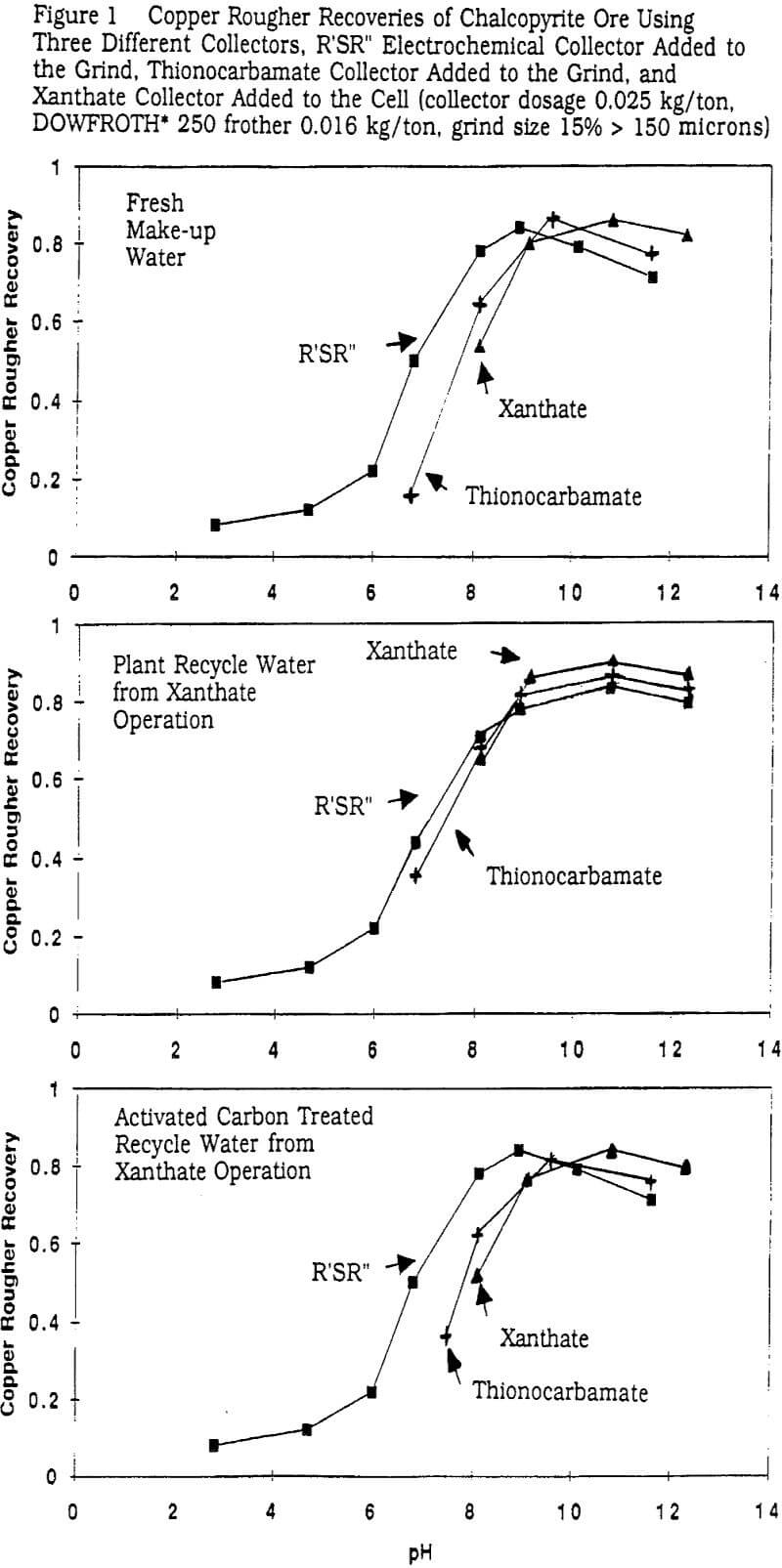
A prime example of this is the separation of chalcopyrite/molybdenite (sulfur rich layer) from pyrite (iron rich layer). Figures 1 and 2 show sets of laboratory data on a chalcopyrite ore of head grade 0.88% Cu and 8.6% pyrite. The collectors involved are the plant standard of potassium amyl xanthate collector, isopropyl ethyl thionocarbamate collector, and R’-S-R” electrochemical collector of the form C2H5SC8H17. The water used in the data graphed in the upper portion of each of these figures was fresh well water which was the make-up water source for the plant operation processing the ore in question. While the fresh water is not soft, 100 ppm Ca++, the organic content was nil.
The maximum Cu recoveries achieved in the fresh water tests with each collector are similar but clearly the shape of the recovery profile as a function of pH is different. The electrochemical collector is operable at a lime loading that is 30% of that of the xanthate with obvious cost savings implications. The thionocarbamate collector gives intermediate results. The main difference, however, between the various collectors is in the recovery profiles of pyrite. The electrochemical collector essentially recovers very little pyrite over the entire basic pH range (<10% of the pyrite recovered by the xanthate). The implications of this are significant relative to flotation throughput, simpler cleaning circuit options, etc.
The copper recoveries are slightly higher (at the same active freshly added collector dosages) with the plant recycle water than with only the fresh water. It is also interesting to observe that all three recovery profiles look almost identical using plant water. It appears that the high organic residual level is controlling the selectivity of the circuit. In other words, the nature of a primarily electrochemically driven process with its inherent potential for selectivity is rather easily overwhelmed by the presence of xanthate and/or related thio containing recycle products. Therefore attempts at getting improved pyrite selectivity through xanthate dosage control or pH control are probably a waste of time and effort unless one controls the residual organics in the water being used.
 |
 |
The bottom portions of Figures 1 and 2 show the same ore and test conditions but now run on the high organics containing plant water that has been subjected to an activated carbon adsorption unit before use in grinding and flotation. The water hardness is not much affected by this treatment but the carbon and sulfur contents of the water both drop below 2 ppm. It is evident that such water treatment is successful in restoring the high pyrite selectivity of the electrochemical collector and also the thionocarbamate selectivity. This particular set of experiments is qualitatively similar to a number of other sulfide mineral plants in which the author has done the same type of experiments.
The technical messages here are important:
1) the use of water soluble collectors and their tendency to reappear in still active collecting forms in closed water recycle systems needs to be closely monitored and controlled if one wants to make significant gains in iron sulfide selectivity against electrochemically influenced minerals; and
2) if one wants to efficiently use an electrochemically driven collector approach, it is important to have plant water available for grinding and flotation;
a) that has been at least partially cleaned up of soluble organics; and/or
b) that has had sufficient residence time in tailings storage for complete organic decomposition to occur; and/or
c) that has had sufficient plant use without soluble collectors on a long enough time basis leading to a low equilibrium level of soluble organics.

