Table of Contents
The Wind or Melting Furnace
Principle.—According to the equation 2PbO + C = Pb2 + CO2, 446 grains of litharge fused with excess of charcoal should yield 414 grains of metallic lead. If the temperature be too high, PbO volatilises, and this loss, which varies with the temperature and air present, can be checked by weighing the button from a given quantity of litharge.
Apparatus.—Wind furnace, tongs, moulds, crucibles (C) and lids, pulp scales, weights, etc.
Reagents.—Litharge and finely ground charcoal.
Details of Operation.—Prepare a low rod fire in the furnace. Adjust the pulp scales. Take 6 pieces of glazed paper, each about 25 c.m. x 30 c.m., and number them 1 to 6. Weigh out 6 charges, each consisting of 10 gm. (or 100 grains) powdered litharge. Place one charge on each of the 6 pieces of paper.
Weigh out 6 charges, each consisting of .5 gm. (8 grains) of finely powdered charcoal, placing one on each of the 6 pieces of paper. Carefully and thoroughly mix each of the 6 combined charges. This may be done either by transfer to a wedgwood mortar or by means of a spatula on the paper. The furnace will by this time require attention, and should be restoked to obtain a dull red heat.
Transfer charges 1 and 2 each to a crucible (Battersea round). On top of each charge, after transference to the crucible, add a cover of 2 gms. (20 grains) charcoal. Place the two crucibles in the furnace, sinking them till the coke is level with their top edges. This may be done either by building the coke up round the crucibles if the fire be low, or by making a cavity by aid of the tongs and inserting the crucible in the cavity; or, better still, two old crucibles (dummies) are built into the coke at the outset, and on removing them the crucibles containing the assays can be introduced into the cavities thus obtained. Place a lid on each crucible. Close the furnace door. Examine every few minutes till the crucibles become dull red, then allow them to remain in the furnace at this temperature for twenty minutes.
Meanwhile, clean two moulds, heat them till uncomfortable to the touch, oil or rub with graphite their interior surfaces. Remove each crucible in turn, and carefully pour into its respective mould. When cool (after five minutes or so), carefully remove the lead from each mould and weigh, taking care to collect any small prills of metal. Enter the results, with notes of the quantities of litharge, charcoal, time and heat of fusion, in your laboratory note-book.
Calculation of Results.—As 446 grams pure litharge should yield 414 grams lead, 10 gms. litharge should yield 10 x 446/414 gms. lead = 9.28 gms. lead. The student will probably obtain buttons weighing about 8 gms., the remainder being lost in fusion. The results, though inaccurate, will be compared with those from the remaining tests. The student must remember that the litharge is rarely pure. Now transfer charges 3 and 4 to crucibles, and insert the charged crucibles in the furnace in the cavities left by 1 and 2. Urge the fire, and when the temperature becomes bright red, allow the assays to remain at that temperature for twenty minutes. Remove, pour, and collect the lead as before. Enter the results in your note-book.
Now proceed with charges 5 and 6, urging the fire to its utmost to produce a white heat. Allow the charged crucibles to remain at this temperature for 20 minutes. Remove, pour, and note the results as before.
The student will find some difficulty at first in regulating the temperature. If he has carefully followed the instructions given, he should obtain three pairs of buttons. The buttons of each pair should not differ more than a few grains from each other. The weights of each successive pair of buttons should diminish as the heat increases after a red heat has been obtained. (Probably the middle pair will give the highest results.)
In reducing lead from litharge by means of charcoal a certain temperature is required before the reaction will take place. Any considerable increase above this temperature can only lead to loss by volatilisation of the litharge. Also, at this ‘certain temperature,’ a certain time is required to reduce all the lead from a given quantity of litharge. The temperature being constant, the time depends largely on the intimacy of mixture of the charcoal and litharge ; this, again, depends on the degree of fineness to which the two substances are ground. As a general rule, the finer the grinding and mixture the better the results. When once this necessary period of heating has elapsed, any further heating can only lead to loss, the lead being liable to reoxidation, though with the excess of charcoal present this loss must be slight.
So far the student has kept the time constant, and has varied the temperature. Time will now be considered.
The Effect of Variations in Time
Weigh out 6 charges as before.
Place 1 and 2 in the furnace and keep at a bright red heat for five minutes. Remove, pour, collect and weigh buttons. Note results.
Place 3 and 4 in the furnace at the same temperature, but for twenty minutes. Treat as before, noting results.
Place 5 and 6 iu the furnace at the same temperature, but for sixty minutes. Again note results.
These results will give the student some idea of the influence of time on an assay. Besides the losses mentioned, others, such as the formation of silicate of lead, occur. This loss may to some extent be avoided by coating the interior of the crucible with graphite. When considering the fire-assay of lead ores the student will see that certain precautions are taken to prevent the formation of silicates of lead. He will see that to obtain the least possible results other charges than that just given are adopted. The experiments just described are given not for the purpose of estimating the lead in litharge, but to show the student the effects of varying temperature and time on au assay.
(b) The Muffle Furnace.
The Effects of varying the Temperature
Principle.—If a weighed quantity of silver be wrapped in sheet lead and cupelled in a muffle furnace, and the resulting button be weighed, any difference in weight (neglecting for the present the small quantity of silver in the lead) represents loss in cupellation. This loss varies with different temperatures.
Apparatus.—Muffle furnace and tools, cupels, balance sensitive to 1/1000 grain, weights, etc.
Reagents, etc.—Sheet lead, test silver.
Details of Operation.—Weigh out three charges as follows, the lead being weighed on the pulp scales, and the silver on the finer balance,
Silver,…………………………………………..0.2 gm. (or 3 grains).
Lead,………………………………………….10 gm. (or 150 grains).
The student will meet with some difficulty in obtaining the exact weight of silver. To save time he may place on the pan approximately .2 gms. and note the exact weight, perhaps .242 gm. or .175 gm.
Wrap each lot of silver in the sheet lead. Place three good dry cupels in the furnace, which should be at a red heat. After a few minutes insert charge 1 in its cupel. Close the door till cupellation commences. Open the door and regulate the temperature, so that feathery crystals of litharge form round the inner rim of the cupel. When finished, carefully remove the cupel, taking precautions to prevent the bead ‘ spitting.’
When cool remove the bead, and free it from bone ash by gentle tapping with a hammer. Weigh the bead and note the result.
Urge the fire, and when the muffle is at a bright red insert charge 2 in its cupel. Continue the cupellation at a bright red, and when finished remove as usual; cool, clean, and weigh. Note the results.
Urge the muffle still further, till almost a white heat is obtained. Insert charge 3. Maintain as nearly as possible a white heat during cupellation. Remove, cool, and weigh as before. Note the result.
For comparison, the student may tabulate the results of these three experiments as follows:—

The figures quoted, though imaginary, indicate to the student the probable results of his experiments, and demonstrate the increase in the loss of silver with increasing temperature.
The student is referred to more advanced text-books for details of experiments regarding other losses, such as absorption of gold and silver by the cupel, etc. Want of time will prevent the student at this stage from following up these researches, and in the following pages brief notes will be given, where necessary , regarding the effect of such losses on the accuracy of au assay, and on the means of reducing such losses to a minimum.
The purpose of the present experiment will be served if the student determine for himself the important part played by temperature in cupellation, From the nature of the case experiments regarding time are not so applicable here as when dealing with the wind furnace.
The Influence of Temperature and Time on the Roasting of Sulphide Ores
Principles of Experiment.—Samples of the ‘roast’ are taken at various stages of the operation, and the state of the desulphurisation and other points of value determined approximately by suitable tests.
Apparatus.—A furnace (preferably muffle), roasting dishes (8 cm.), the usual furnace tools and chemical apparatus for qualitative work.
Reagents, etc.—A sulphide ore containing copper, iron, and sulphur, also the ordinary qualitative chemical reagents.
Details of Experiment.—Raise the temperature of the muffle to a very dull red. Place in the roasting dish about 20 gms. of the ore which has been ground and passed through a 60 sieve. Transfer the dish and charge to the muffle.
(1) When a pale blue flame appears on the surface of the charge remove about 5 gm. (one quarter of the ore). On removal, smell the partially roasted ore and note the smell of burning sulphur (SO2). Shake up these 5 gm. with about 100 c.c. H2O in a small flask. Repeat the shaking several times. Filter, and to a portion of the filtrate add NH4HO in excess. Little or no Cu or Fe will be detected in this filtrate, indicating that, so far, soluble compounds of these elements have not been formed to any extent.
(2) After the removal of sample (1) carefully stir (with a long wire bent at one end) the charge in the furnace. Continue stirring, raising the temperature till the blue flame ceases. Remove a sample as before and treat with water, filtering and adding ammonia as before. The presence of Cu and Fe should be very marked at this stage.
(3) Raise the temperature and continue the roast; after the lapse of 30 minutes remove a small sample and test again for soluble sulphates. Continue the roast, raising the temperature till no reaction is obtained for soluble sulphates. This last stage takes a considerable time. The student may now test by the usual methods the thoroughly roasted ore and ascertain whether traces of sulphur still remain. Unless subjected to repeated roastings at a high temperature with the aid of oxidisers it will always be found that a small amount of sulphur is left in an ore after roasting. When roasting gold ores previous to crucible assay, careless or hasty work on the part of the student will result in the presence of a considerable percentage of sulphur, which in the crucible acts as a reducer. Larger buttons than usual are obtained, the sulphur assisting the charcoal or other reducer used.
If time permit, the student may further experiment on ores containing zinc, arsenic, etc. For the present, however, the average student, whose time is limited, had better strictly confine his attention to the work laid down in these pages.
Estimation of the Reducing Power of Charcoal, Flour, and Argol
Method of Experiment.—The reducing powers are compared by ascertaining the amount of lead which one part by weight of each of these reducers can extract from litharge when subjected to the action of heat in a crucible.
Apparatus required.—As in experiments on the wind furnace.
Reagents. — Finely powdered litharge, charcoal, flour, argol, glass powder, and soda.
Details of Experiment.—
(1) Weigh out two charges of the following—
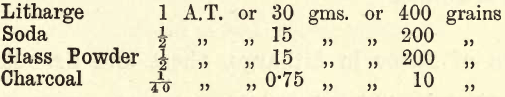
The weights are here given in three systems, but in future only one system will be given, from which, if necessary, the student may obtain corresponding values in other systems by simple calculations. Mix intimately the two charges. Transfer to F crucibles, cover, fuse in a bright red fire till the surface of the charge appears tranquil, i.e., until all frothing has ceased, and the surface of the molten charge is slowly moving in gentle waves. Pour into clean, hot, oiled moulds (preferably conical). Care must be taken in pouring that no lead sticks to the sides of the crucible. The cause of this is generally uneven heating, the top of the crucible projecting above the surface of the coke. The remedy is proper firing.
When cool, remove from the moulds; break away and clean the two buttons, weigh them, and note their weights, which should not diifer more than 5 grains.
(2) Weigh out two charges of the following—

Mix, fuse, pour, clean buttons, and weigh as before. Note the results.
(3) Weigh out two charges of the following—

Treat as before, and note results.
Calculation of Reducing Power.—Three sets of results are obtained, e.g.—

Accuracy of Results.—Considerable practice is necessary in order to obtain much accuracy, but the results obtained in these experiments will be sufficient for the student’s purpose. The results will vary with the quality of the reducer taken. Argol, for example, varies from 5 to nearly 7. In his future work the student will be instructed in certain cases to add sufficient reducer to obtain a button of 20 gms. Supposing the figures quoted represent the student’s results, then either 20/28.5 gms. charcoal, or 20/11.7 gms. flour, or 20/6 gms. argol may be used. In all such calculations decimals may be disregarded, and the nearest unit adopted as a divisor.
Estimation of Silver in Litharge, Sheet and Granulated Lead
Method adopted.—Weighed quantities of sheet or granulated lead are cupelled under certain conditions, and the silver bead obtained is weighed. In the case of litharge, the lead is reduced and then cupelled.
Apparatus.—Wind and muffle furnaces, balance, etc., as before.
Reagents.—As before, with the addition of borax and salt.
Details of Estimation.—In carrying out the following operations, the student must carefully note the conditions under which he is working, as the results here obtained will be made use of in subsequent assays, in which similar conditions must be observed. These remarks apply particularly to cupellation.
Weigh out two charges of the following—

Mix, fuse, pour as usual. Hammer the resulting button into a rough cube. Transfer to a cupel, and carefully cupel at a low temperature producing ‘ feathers.’ Remove the cupel as soon as finished, and weigh the silver bead. This amount of silver must always, therefore, be deducted when 60 gms. litharge are used in a silver assay. Proportionate deductions are made with other quantities of litharge. In order that this deduction be accurate, all buttons should be cupelled as nearly as possible at the same temperature.
In the case of sheet or granulated lead, transfer the 60 gms. charge, without fluxes, to a 2½” scorifier, and when the eye has closed in, pour, and then cupel the button. Weigh the bead, and the necessary deduction is obtained as before.
