Table of Contents
In the manufacture of gun forgings and other steel parts that, in service, are subject to sudden high stresses and shocks, it is most desirable to use steel possessing the greatest toughness and ductility possible without sacrifice of strength. In order to obtain this condition, it is necessary to procure steel that shows the highest possible elongation and reduction of area without lowering the tensile strength and elastic limit. Proper heat treatment of the steel can control this condition within certain limits. When heat treatment has failed to produce the desired results, metallurgists have used steels containing molybdenum, zirconium, vanadium, chromium, tungsten, etc.
The purpose of this paper is to describe a method by which these desired physical properties may be procured—by the elimination of certain impurities that inherently exist in steel made by the open-hearth process, and without the use of expensive alloys.
DESIGN OF GUN FORGINGS
In the manufacture of gun forgings, a certain elastic limit is fixed by the designer, and the walls of the gun are made of the proper thickness, allowing a suitable factor of safety for the high stresses and sudden shocks that occur during gun firing. The elastic strength of the gun is about 1.4 times the stress set up at any point along the bore of the gun during firing with the maximum powder charge.
As the stresses set up in the walls of the gun during firing are mostly “tangential,” all physical tests are taken in this direction. Due to the length of the forgings, these tangential test bars are always taken at right angles and transverse to the direction of flow of the metal in forging. Furthermore, test bars taken across the grain of the metal will more frequently expose defects and foreign inclusions in the steel than will bars taken in the direction of flow of the metal in forging. Impurities in the steel will also be more readily detected by transverse test bars.
With a given tensile strength and elastic limit, a steel with higher elongation and reduction of area is more desirable for service where sudden stresses and great shocks are encountered. The high elongation denotes ductility and the high reduction of area denotes toughness; for the purposes just mentioned, these properties are preferable to a higher tensile strength and higher elastic limit with a lower elongation and lower reduction of area.
RESULTS OBTAINED WITH ELECTRIC STEEL
Some interesting results were obtained recently in the manufacture of heavy ordnance forgings at the U. S. Naval Ordnance Plant, South Charleston, W. Va. The steel was made by the duplexing process, in which cold charges of pig iron and scrap were melted in a 75-ton basic open-hearth furnace, where, the dephosphorizing was done, and subsequently the deoxidizing and desulfurizing was done in two 40-ton basic- lined electric furnaces. A comparison of the physical results obtained from forgings made by this process with similar forgings made directly in an acid open-hearth furnace at the works of one of the leading industrial steel plants, noted for high-grade open-hearth steel, shows that the steel with the lower phosphorus and sulfur has the greater toughness and ductility. Transverse tensile test bars from electric steel, though having the same tensile strength and elastic limit as the bars from the open-hearth steel, have a much greater elongation and reduction of area.
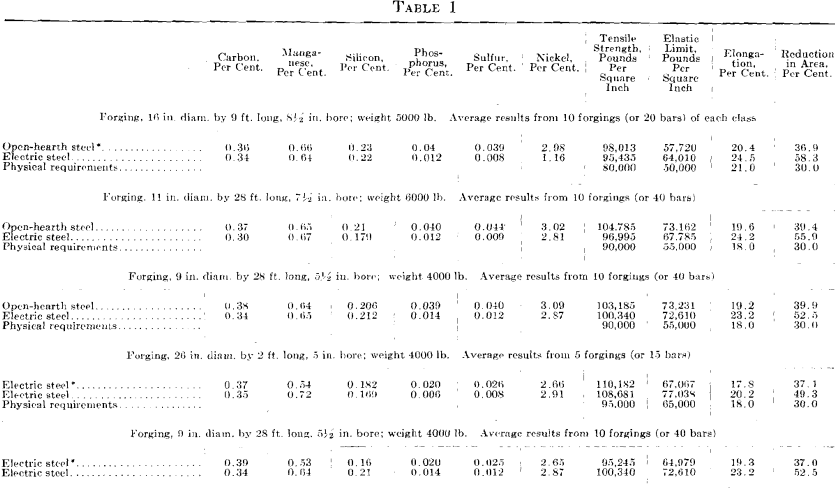
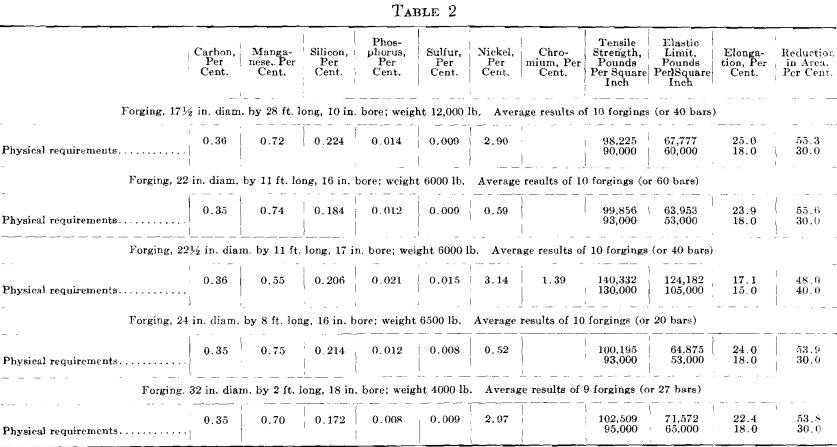
The results given in Tables 1 and 2 were obtained on tangential tensile test bars. All forgings received a green annealing before machining; after machining they were quenched and drawn. For the results given in Table 2, it was impossible to procure comparative results from open- hearth or electric steel made in private industrial plants. The first impression on comparing these results is that the higher elongation and reduction of area are due to the low phosphorus and sulfur, as shown by the chemical analysis; all the other elements are approximately alike.
EFFECT OF PHOSPHORUS
It would be difficult to draw any comparison between the open-hearth and electric steel in regard to the phosphorus content. This element is in solid solution with the iron as a phosphide and the percentages are too small in both the open-hearth and the electric steel to denote any difference even with a microscope. The lower phosphorus in the electric steel might have a slight effect on the elongation, due
to producing a somewhat smaller grain and decreased brittleness in the steel.
EFFECT OF SULFUR
It has been stated by a recognized authority on the manufacture of steel that “the effect of sulfur on the cold property of steel has not been accurately determined but it is certain that it is unimportant, in common practice, the content varies from 0.02 to 0.10 per cent, and, within these limits, it has no appreciable influence on the elastic ratio and the elongation or the reduction of area.” This statement probably relates to commercial steels tested longitudinally. In this case the sulfur, in the form of manganese sulfide, has been drawn out into thin shreds in the direction of forging or rolling and is not so easily noticed in the results of longitudinal test bars as it would be in the case of transverse bars. With the overbalancing amount of manganese present in all the steels referred to in this article, probably no iron sulfide is present in the steel for none of the ingots showed any signs of tearing during forging. Steel containing iron sulfide is known to tear in forging and is termed “hot short” by steelworkers. Manganese sulfide has been described as being present in the ingot in the form of small globules between the grains of the metal. Having about the same fusing point as the metal, these inclusions become equally plastic when the ingot is heated for forging and are drawn out into long thin shreds—just as slag is drawn out in
wrought iron. If, however, the amount of manganese sulfide present is not enough to form these globules, these shreds will not be developed in forging and transverse test bars will show as good results in elongation and contraction as longitudinal bars.
The following test bars were taken from a piece of steel resembling that shown in Fig. 3. Two of the bars were drilled longitudinal with the forging and the other two tangential. They were given exactly the same
heat treatment, quenched at 1425° F., and drawn at 1200° F.; the results were as follows.
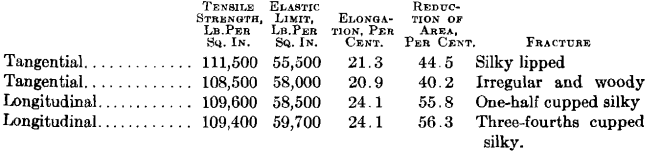
It will be noted that the tensile strength and elastic limit are practically equal in the tangential and longitudinal bars, but the non-metallic
enclosures in the steel caused the tangential bars to show a lower elongation and lower contraction than the longitudinal bars.
EFFECT OF OXYGEN
There is nothing in the usual chemical analysis to show how much oxygen is present in steel. It exists in small amounts in even the best steel and has bad results. In large amounts, it produces tearing during forging or rolling, and when cold is brittle under shock. If present in steel, it is probably in the form of iron and manganese oxides and silicates. Oxygen is most prevalent in basic open-hearth steel. Where non-metallic enclosures are great enough to analyze, they have been reported to show the following composition: SiO2 50 per cent., MnO 30 to 40 per cent. Al2O3 7 to 18 per cent., FeO trace. In the acid open-hearth furnace a more effective reaction between the slag and the steel tends to deoxidize the steel more thoroughly. This cannot be done completely on account of the air present in the acid open-hearth furnace.
A condition exists in the basic electric furnace which cannot exist in the basic or the acid open-hearth furnaces. With a reducing atmosphere in the furnace, it is possible to form a calcium carbide slag free from metallic oxides; with constant rabbling of the bath, any oxides in the steel will rise to the slag, where they are reduced by the carbon present. The iron and manganese are returned to the bath, and the resulting carbon monoxide is liberated to the atmosphere of the furnace. Unless the slag and bath are free from oxygen, it would be impossible to maintain a carbide slag; and unless the slag and bath were thoroughly deoxidized, it would be impossible to retain the sulfur in the slag as calcium sulfide.
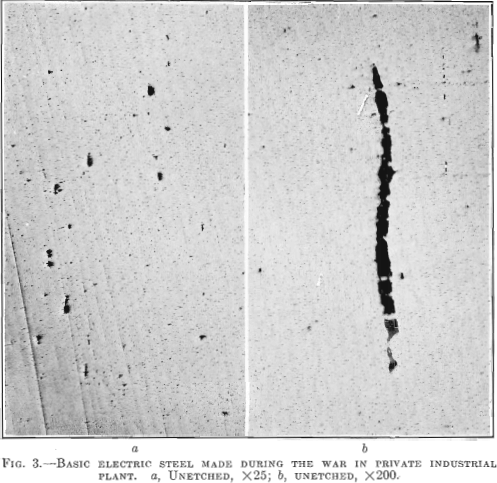
Hence, the conditions that bring about the elimination of sulfur from the steel guarantee that oxides and other non-metallic impurities have also been eliminated. This is demonstrated in the case of the last two forgings in Table 1. While the forgings were made by the electric refining process in different steel plants, the test bars of steels containing the higher sulfur show no better results than the forgings made by the acid open-hearth process. The photomicrograph of the electric steel with high sulfur, Fig. 3, reveals more enclosures than the electric steel with low sulfur.
PHOTOMICROGRAPHS OF OPEN-HEARTH AND ELECTRIC STEEL
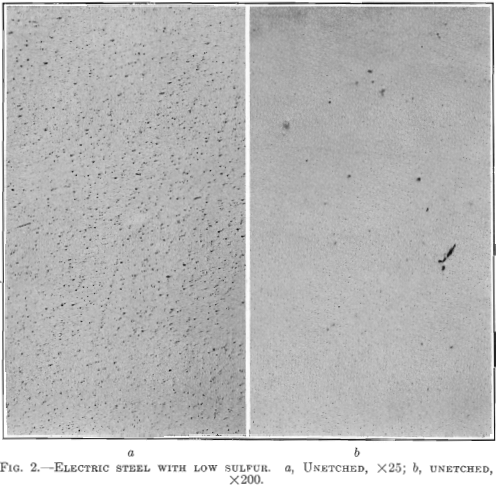
Photomicrographs have been taken of bars picked at random from each of the three classes of steels described. The polished surface of these bars was parallel to the direction of forging. One set of photomicrographs was taken at 25 diameters, so as to include as many enclosures as possible and to show their distribution. A second set was taken at 200 magnifications, to show the formation of the enclosures. It will be noted that the non-metallic enclosures in the steel with low sulfur and highest elongation and reduction of area are smaller than in the steel with higher sulfur content and the lowest elongation and reduction of area. All the enclosures in the low-sulfur steel seem to be of the same kind, whereas, in the acid open-hearth and electric steel with higher sulfur, there are two types—the small round ones and those that were
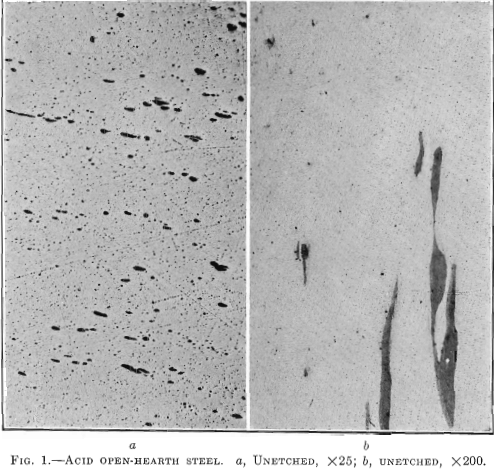
elongated and drawn out in forging. The light colored elongated enclosures in the acid open-hearth steel are probably a mixture of manganese sulfide, oxides, and silicates.
Fig. 1 shows a longitudinal section taken from an acid open-hearth steel forging 11 in. in diameter, 28 ft. long, 7½ in. bore, weighing 6000 lb., having the following chemical analysis: Carbon 0.35 per cent., manganese 0.68 per cent., silicon 0.240 per cent., phosphorus 0.040 per cent., sulfur 0.053 per cent., nickel 3.00 per cent. Physical tests on tangential test bars gave the following results:
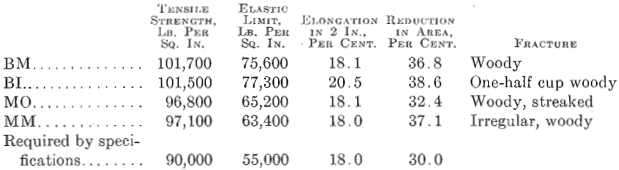
This forging was green annealed at 1600° F., rough machined and quenched at 1450° F., and drawn to 1200° F. The elongated enclosures or shreds follow the direction of forging. A tensile bar taken transversely, or across, these enclosures will show a lower elongation and lower reduction of area than if these impurities were not present. No aluminum was added to this steel in the open hearth. This enclosure is much lighter than that in the electric steel. It did not pull apart in forging and appears to have been more plastic and probably contains more manganese sulfide than either of the other two specimens, Figs. 2 and 3.
In Fig. 2 is shown a longitudinal section taken from a low-sulfur electric steel forging 11 in. in diameter, 28 ft. long, 7½ in. bore, weighing 6000 lb,, having the following chemical analysis: Carbon 0.28 per cent., manganese 0.74 per cent., silicon 0.140 per cent., phosphorus 0.011 per cent., sulfur 0.006 per cent., nickel 2.81 per cent. Physical tests on tangential test bars gave the following results:
This forging was green annealed at 1600° F., rough machined and quenched at 1450° F., and drawn to 1190° F.
The elongated enclosures found in the acid open-hearth steel are lacking. Transverse test bars show high elongation and high reduction of area with no decrease in tensile strength and elastic limit. No aluminum was added to this steel in the melt shop.
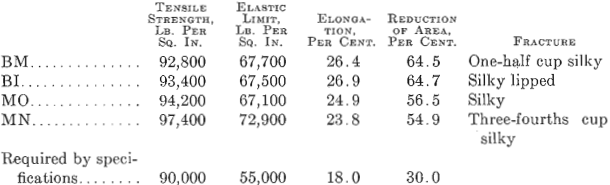
Fig. 3 shows a longitudinal section taken from a basic electric steel forging 9 in. in diameter, 28 ft. long, 5½ in. bore, weighing 4000 lb., having the following chemical analysis: Carbon 0.41 per cent., manganese 0.48 per cent., silicon 0.168 per cent., phosphorus 0.026 per cent., sulfur 0.029 per cent., nickel 2.60 per cent. Physical tests in tangential test bars gave the following results:
This forging was green annealed at 1600° F., rough machined and quenched at 1450° F., and drawn to 1220° F.
Elongated enclosures similar to those found in the acid open-hearth steel are equally prominent but not so numerous. They are darker in color and the fact that they pulled apart indicates that they are more brittle. These inclosures are probably silicates of iron, manganese or aluminum. From 4 to 6 oz. of aluminum per ton of steel was added to the bath in the electric furnace, on the end of a rabble iron, just before tapping.
MANUFACTURE OF STEEL AT NAVAL ORDNANCE PLANT
From the foregoing physical results and substantiating data, it is evident that the presence of sulfur, oxides, and other non-metallic enclosures are detrimental to the ductility and toughness of steel. Where the best quality of steel is required, it is necessary to keep these impurities to a minimum. The basic open-hearth furnace eliminates the phosphorus but only slightly reduces the sulfur; the oxides must be eliminated by the addition of deoxidizers, such as ferromanganese, ferrosilicon, aluminum, etc., which are sometimes added to the open-hearth furnace and rabbled after the air is shut off, but more frequently are added to the metal in the ladle. If added in the ladle, the reactions are incomplete and the products of combustion remain suspended in the steel, forming harmful non-metallic inclusions. Gun forgings and other ordnance material, where transverse tests are required, have never been successfully made from basic open-hearth steel. The bars generally fail in elongation and reduction of area tests, because of the presence of these inclusions. They generally break with a laminated and woody fracture.
The acid open-hearth furnace is better for making steel free from oxides and non-metallic impurities, and ordnance forgings have been obtained from acid open-hearth steel. While neither phosphorus nor sulfur can be eliminated in this furnace, the amount of these impurities may be kept down by the selection of high-grade scrap and pig iron. The oxides may be largely eliminated by the effective reaction between the slag and the steel.
The method of making steel at the U. S. Naval Ordnance Plant aims for the elimination of phosphorus, sulfur, and oxides. The metallic charge of the open-hearth furnace consists of 40 per cent, basic pig iron and 60 per cent, miscellaneous scrap, including turnings, crop ends, etc.; up to 8 per cent, of limestone is added with the charge and sufficient ore to lower the carbon to approximately 0.20 to 0.25 per cent., which is slightly below the amount required in the finished steel.
The pig iron has the following approximate analysis: Carbon 3.50 to 4.00 per cent., manganese 0.95 per cent., silicon 0.65 per cent., phosphorus 0.15 per cent, and sulfur 0.04 to 0.05 per cent. The crop ends are usually low in phosphorus and sulfur, being the discard from ingots made at this plant by the electric refining process. The miscellaneous scrap, consisting of boiler plate, punchings, and turnings, averages approximately 0.04 to 0.05 per cent, phosphorus and sulfur.
The limestone shows the following analysis: SiO2 1.43 per cent., Fe2O3 + Al2O3 0.60 per cent., CaCO3 97.54 per cent. A small quantity of fluorspar added to the slag has a negligible effect upon the analysis.
The average analysis of the final slag taken from the open-hearth furnace on nineteen consecutive heats just before tapping is as follows: SiO2 15.43 per cent., FeO 19.27 per cent., Al2O3 3.66 per cent., MnO 8.02 per cent., CaO 45.02 per cent., MgO 6.37 per cent., P2O5 1.80 per cent., S 0.031 per cent.
The average analysis of the steel prior to tapping these same heats was as follows: Carbon 0.23 per cent., manganese 0.26 per cent., silicon 0.010 per cent., phosphorus 0.007 per cent., sulfur 0.015 per cent., nickel 0.66 per cent., chromium 0.00 per cent. After it is tapped from the open- hearth furnace into a 75-ton ladle, the steel is teemed through a 2½ in. nozzle into two 40-ton basic electric furnaces for deoxidizing and finishing. Usually 2 lb. of 50-per cent, ferrosilicon and 3 oz. of aluminum for every ton of steel tapped from the open hearth are added to the ladle, to take up any oxygen present, which might lower the carbon content while the steel was in the ladle.
An average analysis of the slag left in the ladle from the nineteen heats, after teeming into the 40-ton furnace was as follows: SiO2 17.27 per cent., FeO 18.32 per cent., Al2O3 5.75 per cent., MnO 7.84 per cent., CaO 42.79 per cent., MgO 8.15 per cent., P2O5 1.65 per cent., S 0.028 per cent.
The average analysis of the metal as teemed into the electric furnaces from the open hearth on the same heats was as follows: carbon 0.20 per cent., manganese 0.23 per cent., silicon 0.037 per cent., phosphorus, 0.007 per cent., sulfur 0.016 per cent., nickel 0.67 per cent., chromium 0.00 per cent. Both the furnace and the ladle slags show a slight increase in silica and alumina, which may be caused by the addition of the ferro-silicon and aluminum into the ladle. Some of these same elements may have been washed from the sides of the ladle and floated up into the slag. A comparison of the steel analysis will show that the silicon increased slightly; also, that there was a slight drop in carbon and manganese while the steel was in the ladle. The average phosphorus content of the charge into the open hearth was about 0.08 per cent., because of the comparatively low phosphorus in the crop ends.
After teeming the molten open-hearth steel into the 40-ton electric furnaces, a new slag is made up of burned lime, fluorspar, and ground coke. This represents, in weight, about 3 per cent, of the metal charged and is added from time to time, depending on the condition of the bath and the consistency of the slag. The operation from now on is a deoxidizing one. Under normal conditions, the bath is held from 3 to 5 hr. in a reducing atmosphere. The burned lime used in making up the new slag from the electric furnace showed the following analysis: SiO2 2.50 per cent., Fe2O3 + Al2O3 1.50 per cent., CaO 68 per cent., MgO 20 per cent.; loss on ignition 8 per cent. The MgO content is higher than usual, but a local lime is used for economic reasons; regular practice limits the MgO content to 5 per cent.
The final slag from the electric furnaces, after finishing the steel, showed the following approximate analysis on six consecutive heats:
The high MgO in these slags comes from the high MgO in the lime used. The low sulfur is caused by the low sulfur in the charge. The high basicity of this slag and its freedom from metallic oxides, compared with the slag from the open-hearth furnace, should be noted. Unless the slag and steel are thoroughly deoxidized, it would be impossible to form the carbide slag; without such a slag, it would be impossible to eliminate the sulfur from the steel.
The finished steel on these same six heats showed the following analysis:
The low sulfur in the final slag denotes the possibility of removing a greater amount from a steel of higher sulfur content from the open hearth. This would result from using a lower grade of scrap and pig iron with higher sulfur content in making up the charge for the open- hearth furnace. This might occur in plants where miscellaneous scrap is purchased in the open market and where crop ends are not so low in sulfur as those at this plant.
DESCRIPTION OF REACTIONS
The reducing atmosphere and high temperature existing in the electric furnace permit the following reactions: When the molten steel comes from the open hearth, it contains oxides, principally in the form of FeO and MnO. As the MnO is less soluble in the molten metal than the FeO, it rises to the slag, which has been added on top of the steel as teemed into the electric furnace. This slag contains CaO and C. As the MnO rises to the slag, the following occurs:
MnO + C = Mn + CO (1)
The Mn goes back to the bath and the CO is liberated as a gas. When the Mn goes back to the bath, the following reaction takes place:
FeO + Mn = Fe + MnO (2)
Reaction (1) again takes place. When this reaction is complete and all metallic oxides eliminated from the slag, another reaction between the lime and carbon takes place, in which calcium carbide is formed:
CaO + 3C = CaC2 + CO (3)
This reaction, however, will not be stable as long as there is a migration of either MnO or FeO from the bath to the slag. In this case, the following occurs:
CaC2 +3MnO = CaO + 3Mn + 2CO (4)
or
CaC2 + 3FeO = CaO + 3Fe + 2CO (5)
As long as the slag retains its carbide condition after repeated rabbling, it may be assumed that the bath has been freed from oxides. When reaction (3) becomes stable, it is possible to eliminate the sulfur from the steel.
The sulfur is present as FeS and MnS. As calcium has a greater affinity for sulfur than either Fe or Mn, its presence in the slag causes the following reaction:
3FeS + CaC2 + 2CaO = 3Fe + 3CaS + 2CO (6)
and
3MnS + CaC2 + 2CaO = 3Mn + 3CaS + 2CO (7)
The Fe and Mn return to the bath, the CO goes off as a gas, while the CaS is held in the slag. If for any reason the slag should take up any metallic oxide, the conditions expressed in equations (6) and (7) would be reversed and the calcium would give up the sulfur as follows:
CaS + FeO = FeS + CaO (8)
or
CaS + MnO = MnS + CaO (9)
A simple test for the presence of CaC2 in the slag may be made by sprinkling water on the slag, when acetylene gas will be given off as a result of the following reaction:
CaC2 + H2O = CaO + C2H2
CONCLUSIONS
The manufacture of ordnance forgings from electric steel is not an innovation. It was tried, during the war, in a number of plants. Some of these plants were not so successful as others, probably because the managers were not sufficiently experienced in the many other phases of manufacture necessary in the making of ordnance forgings.
Steel made in an electric furnace will not be of the best quality unless all operations and reactions are preformed completely and satisfactorily. Electric steel with its greater freedom from oxides and non-metallic impurities is more uniform, more homogeneous, and more dense than ordinary open-hearth steel, and if it is cast at too high a temperature or chilled beyond a certain point in the mold, incipient cracks will develop. These minute cracks are radial and are most frequently found near the center of the ingot or forging. Numerous electric-steel plants working on ordnance material, during the war, were troubled with these defects, which from their physical appearance in test bars were called “snow flakes.”
A diversity of opinion has always existed between the leading ordnance steel plants regarding the method of teeming steel into the molds.
Some have consistently adhered to bottom pouring while others have claimed better results from top pouring direct, or through funnels or boxes. The method apparently makes little difference if the steel is placed in the mold at the proper temperature and has been properly cleansed before teeming. Top pouring direct obviates the danger of getting runner brick into the ingot, which frequently occurs in the case of bottom pouring. It also obviates the danger of sand washing into the molds with the metal from the funnel or box. Bottom pouring will give a better surface on the ingot and for some purposes may be more desirable.
DISCUSSION
HARRY T. MORRIS, Bethlehem, Pa.—Was this armor plate forged in both directions? Has the author experimented frequently with transverse and longitudinal bars from armor plate in which the forging was done in both directions? Has he found that irrespective of the content of sulfur, up to 0.06 per cent., the forging in both directions, for a time in one direction and for another time at right angles, produces reasonably consistent and comparable results in the tensile bars, both transverse and longitudinal?
WILLIAM J. PRIESTLEY.—An examination of the photomicrographs of the armor plate made of electric steel will show that the inclusions are approximately the same magnitude in the bars taken in both directions, and that they are smaller than the enclosures in the other armor plate. It would be expected that when the plate was forged in two directions, the inclusions would be drawn out in both directions and they would be more apparent than in the case of the longitudinal bar taken from the plate forged only in one direction.
Figs. 4 and 5 are photomicrographs taken from transverse and longitudinal test bars from a heat-treated armor plate of foreign manufacture, which showed very poor results in ballistic tests. Fig. 4 was taken parallel to the direction of forging the ingot and shows the non-metallic enclosures in the steel elongated. The physical properties of this bar, taken at right angles to the direction of forging, showed the following characteristics: Tensile strength 111,000 lb. per sq. in.; elastic limit, 55,000 lb. per sq. in.; elongation, 13.4 per cent.; contraction, 23.9 per cent.
Fig. 5 was from a longitudinal test bar taken parallel to the direction of forging the ingot; it will be noted that the impurities are round and are cross-sections of the enclosures as shown in Fig. 4. The results of the physical tests on this bar were as follows: Tensile strength, 100,000 lb. per sq. in.; elastic limit, 68,000 lb. per sq. in.; elongation, 26.5 per cent.; contraction, 65.5 per cent.


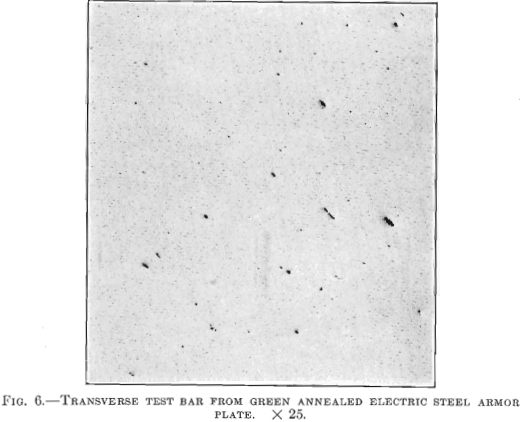

Figs. 6 and 7 are from transverse and longitudinal test bars taken from an armor plate made of electric steel from which the oxides and non-metallic enclosures have been removed. The results of physical tests on the transverse test bar were: Tensile strength 101,500 lb. per sq. in.; elastic limit, 61,000 lb. per sq. in.; elongation, 26.5 per cent.; contraction, 65.8 per cent. The longitudinal test bar, Fig. 7, was taken from the same plate as the transverse bar and showed the following physical properties: Tensile strength, 104,000 lb. per sq. in.; elastic limit, 57,000 lb. per sq. in.; elongation, 25.3 per cent.; contraction, 63.5 per cent.
The forgings from which the bars shown in Figs. 6 and 7 were taken were only green annealed, whereas the plates from which the other bars were taken were heat treated. It will be noted that the electric steel, which is free from non-metallic inclusions, has practically the same strength and ductility in two directions, whereas the steel shown in Figs. 4 and 5, which contains a greater amount of non-metallic inclusions, has strength and ductility only in one direction and is very weak and lacks toughness and ductility in the other direction.
C. P. PERIN, New York, N. Y.—I did not understand that was the question; I thought Mr. Morris asked as to the deleterious effect of sulfur, although the steel were forged in both directions, whether it would have a bad effect.
HARRY T. MORRIS.—I asked about the physical results, not the photomicrographs. It is not to be disputed that if the sulfur content is three times as high in one piece of steel as in another, the microscope might show more inclusions in a given area where the sulfur is high; but the thing that I wanted to show was that it does not necessarily follow that specimens from a piece of steel containing 0.04 per cent, or more sulfur will not give practically equal physical properties in both directions; that is, there is some treatment besides the elimination of sulfur that will give it equal physical properties in both directions.
W. J. PRIESTLEY.—We have tried to heat-treat foreign inclusions out of steel. Some melting men say that it can be forged out and some say that it can be heat-treated out, but I have never seen it done, nor have I seen it broken up or scattered.
HARRY T. MORRIS.—The question of sulfur in proportions up to 0.04, 0.05, 0.06 per cent, is an important one in all kinds of steel, and there are differences of opinion among engineers, producers, and open-hearth men as to the effect of sulfur up to 0.05 or 0.06 per cent. Some engineers, open-hearth, and electric-furnace people, and steel producers will not admit that a steel with 0.06 per cent, sulfur is not necessarily an inferior steel.
This paper seemed to indicate that if an armor plate, for instance, had 0.05 or 0.06 per cent, sulfur (such sulfur is not shown in this paper but the inference might be drawn by those not familiar with this subject) it would not be quite as good as one with 0.03 per cent, sulfur. I do not believe that the actual resistance in a firing test (where projectiles are fired against armor plate) will demonstrate that a 0.06 per cent, sulfur armor plate is necessarily poorer than one with a 0.03 per cent, sulfur content.
JAMES H. GRAY, New York, N. Y.—When I first read this paper, I thought the author had found a decided difference in the quality of the steel, getting transverse tests practically equal to longitudinal tests in gun steel forged in only one direction. It seemed as if there was a difference between 0.025 per cent, sulfur in certain electric steels and under 0.01 per cent, in the author’s steel. The author seems to prefer low sulfur because it is an indication that he has properly made the steel in the electric furnace; that is, he could not desulfurize the steel without having previously cleaned and deoxidized it. If he could express that idea, it would perhaps clear up the difficulty as to the real value of lower sulfur.
W. J. PRIESTLEY.—In order to reduce sulfur from steel down as low as 0.008 or 0.006 per cent., the steel must be thoroughly deoxidized. A carbide slag is necessary, to take out the last traces of sulfur. I do not want to convey the idea that it is the sulfur that is doing the harm, but if the sulfur can be brought down that low, you must get all the oxides out of the steel.
W. P. BARBA, Philadelphia, Pa.—The author is discussing the manufacture of this product in an electric furnace only. Mr. Morris questions the initial and final content of sulfur, irrespective of the process used to melt and produce the ingot. You have, therefore, two different premises, which cannot be compared without qualification.
There is in armor plate a third dimension, that is from face to face through the plate; if, in my works practice, I saw such a test bar with a cup fracture, I would cut a whole section through the plate and study that macrostructure in order to find out whether or not it had the necessary resistance properties. Because of the successive laminations, exactly like the successive pages of a book, were a projectile fired against the plate, it would meet sheet after sheet of resisting metal, the values of which are not exhibited by a pulled bar. The pulled bar was used to determine if the works superintendent had gotten his grain-size into the most satisfactory condition to resist the impact of a shell at the proving grounds; but he does not want to see a cup fracture in all three dimensions on his plate, therefore, I do not give too much value to cup fractures. As Mr. Morris pointed out, the plate could have been forged in two directions, while in the slab or ingot, form, and by forging thus you can get practically equal test, bars. There is an entirely different set of conditions in the plate to observe from face to face; yet, the plate showing in that third dimension test bar 1 per cent, stretch might keep out a shot.
W. J. PRIESTLEY.—The first two plates made of electric steel had the laminated structure, with fissures between, about which Colonel Barba has spoken. We thought that if we could not improve on that, we were not going to improve our armor plate. We wanted a plate that was homogeneous in structure with a perfect continuity of metal through the thickness, exactly as in the other dimensions. That was obtained from clean steel free from oxides by preventing the ingot from cooling in the mold and by preventing the plate from cooling down after forging. The steel was kept hot all the way through and eliminated that structure. Unfortunately, we did not have time to get a tensile-test bar out of the third dimension, but from physical appearances the structure was homogeneous and lacked that woody formation with fissures generally found in armor plate made in the basic open-hearth furnace. The last plate I saw tested ballistically did not have these fissures; the back of the plate opened up like an orange peel. This plate was rated high. On plates with this laminated structure I have seen a perfect disk pushed from the back in firing tests. It has been my experience that a plate that will open up in the back like an orange peel and hold together on the perimeter of the impact is stronger and better than a plate that will fail by a straight, flat button going out of the back of the plate.
HAAKON STYRI, Philadelphia, Pa.—I have never obtained any satisfactory explanation as to why some ingot molds are made corrugated with sharp corners and others with round corners, or some with six corrugations, some with eight, and some with more. Some plants get good results with sharp-cornered molds and others get better results with round corners.
In Howe and Barba’s paper, an analogy was drawn with cylindrical and corrugated drain pipes, the latter not bursting when the water would freeze. But water expands on freezing and steel contracts. To resist liquid pressure the cylindrical form would be stronger than the corrugated. Is not the explanation of various successes with different ingot molds rather the adaptation of such molds that will best suit the pouring practice at the particular plant or some variation of the practice to fit the mold, so that the resulting skin of the ingot will be strong enough and not burst.
W. P. BARBA.—Doctor Howe’s philosophy on the reported practice, supported by sketches, blueprints and experience dating back to 1897, will answer the questions just raised. The practical point is that about ten factors of design and of practice are brought into consideration.
FRANK D. CARNEY, New York, N. Y.—With the octagon sharp-cornered mold, Fig. 8, when the dentritic or columnar crystals start to grow in from the concave sides they produce a plane of weakness at each corner, trapping the impurities, and it requires great skill in pouring and temperature control to prevent a plane of weakness at the corners.
Our experience at the Bethlehem Steel Co. was similar to Mr. Priestley’s. Sharp-cornered octagon ingots sometimes gave poor transverse tests if test specimens crossed at this point. If the octagon mold was designed with round corners, we did not have the trapping effect in the corners. This type of mold is fool-proof as it allows a wider margin in rate of pour and pouring temperatures. This ingot could be rolled with good results on a blooming mill.
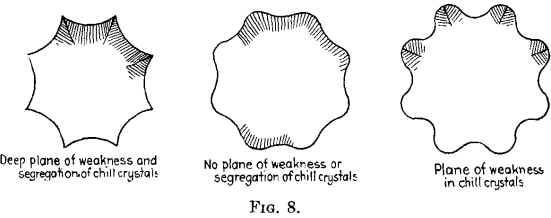
For heavy ordinance work our practice was to slice the ingots in a lathe, bore out the center and inspect the end surfaces, from time to time, with sulfur prints. We never found planes of weakness in the round- cornered ingots and therefore abandoned the use of sharp-cornered molds.
W. P. BARBA.—Mr Carney has correctly shown the two extremes that lie in those two forms of ingot molds. I was, to some degree, familiar with the experience of the Bethlehem Steel Co., and have noted exactly the same thing. If the correct relation is had between cross-sectional areas, the radius to what we call the flat and the radius to what we call the corner, none of the troubles that Mr. Carney mentions, will be experienced. It is a phase through which one must feel his way, and when the right method is found the wrong one should be abandoned.
W. L. HARBRECHT, Niagara Falls, N. Y.—The author says that he saw flakes only in electric steel; I have seen them in both the acid open- hearth and electric steel, and more frequently in the latter. By electric steel I mean that made by the triplex process.
W. J. PRIESTLEY.—I have seen so-called flakes in acid and basic steel. I have examined the defects closely and have found that in the center of the flake, in the acid or basic open-hearth steel, there would be a slag inclusion as a rule; but my experience has been that with the typical electric steel flake there is no foreign inclusion.
W. L. HARBRECHT.—We have examined flakes from both kinds of metal and we did not find any inclusion in either, except that at the edge of the flake, by making a macroetch, we sometimes would see a small dotted line of inclusions along the outside of the flake.
C. P. PERIN.—Was that characteristic of the electric or of the open-hearth steel?
W. L. HARBRECHT.—Of both. When examined with a microscope, we did not see any difference in structure or in any other way.
GEORGE F. COMSTOCK, Niagara Falls, N. Y. (written discussion).— This paper is of great interest, showing as it does the detrimental effects of sulfides and other non-metallic inclusions on the quality of steel. It might be suggested, however, that segregation has an important influence on the degree of harm occasioned by a given amount of non-metallic matter included, because the arrangement of a number of particles in groups or streaks, as is suggested by the photomicrographs in Fig. 1 and Fig. 3, undoubtedly gives a much more serious effect than would a uniform distribution of the same amount of material. Probably one reason for the success attained at the Naval Ordnance Plant in making this steel was the care and time available for insuring a complete absence of segregation, such a condition being practicable at this plant on account of the lack of necessity for a large tonnage of output in a given time.
It does not seem correct to state that oxides are “eliminated by the addition of deoxidizers, such as ferromanganese, ferrosilicon, aluminum, etc. ” Oxygen and iron oxide are doubtless eliminated by these reagents, but at the cost of producing other oxides in the steel, the net result being a formation of oxides rather than their destruction. The oxide of aluminum, especially, is difficult to eliminate from steel in which it has once been formed, and it is nearly always possible to identify steel in which it has been used, by a microscopic search for alumina particles in a polished section. The deoxidizer least liable to contaminate steel with its oxidation products is ferro carbon-titanium, which is not mentioned. Titanium oxide has been found to render slags and oxidation products of other deoxidizers more fusible, and the carbon in this alloy assists in the production of a lively reaction in the steel, which is entirely completed in the ladle in a very reasonable time. No noticeable contamination of the steel by oxides is evident in steel properly treated with this alloy, and an exhaustive series of tests in rail steel has furnished unquestioned proof of its effectiveness as a preventive of serious segregation. It is unfortunate that this method of treating basic open-hearth steel has not been given a thorough trial in the manufacture of ordnance material, for the lack of success from such steel might be overcome, in this way, at much less expense than by the method of duplexing with an electric furnace.
E. J. JANITZKY, South Chicago, III. (written discussion).—The author states that usually 2 lb. of 50-per cent, ferrosilicon and 3 oz. of aluminum are added to the ladle for every ton of steel tapped from the open-hearth, to take up any oxygen present, which might lower the carbon content while the steel was in the ladle. These additions appear unexcelled as deoxidizers as no gases are developed in their reactions but as they hinder the expulsion of occluded gases from the metal, their use is questionable.
The products of oxidation of silicon and aluminum have ample time during the refining period to enter the slag, which is no reason for discriminating against their use. Ferrosilicon and aluminum will kill the steel, in other words, eliminate the liveliness of the steel bath, hence they will not allow a gradual natural dead melt.
A ferromanganese addition will counterbalance supposed carbon loss. Manganese is one of the main deoxidizing elements in the electric-furnace process and the loss of metallic manganese in the ladle would in all probability counterbalance the certain loss of aluminum and silicon, neither of which has any influence on the physical properties of the steel nor takes part in the deoxidizing reactions, as does manganese. Otherwise a higher initial carbon content of the open-hearth melt or the addition of some anthracite to the ladle would be a less expensive way and would also offset the carbon loss. Either anthracite or ferromanganese will take part in the deoxidizing reaction, but when pouring into the electric furnace, the opportunity of gas expulsion is given, whereas silicon and aluminum would hinder it. As degasification cannot be performed with the addition of ferro-alloys, the refining of the bath must take care of it. The degree to which it takes place is a matter of speculation.
The influence of occluded gases on the physical properties of steel has not been thoroughly studied. Recent experiments, however, seem to show that their influence is greater than we assume. Mars and others have pointed to the improved quality that can be obtained by cooling the bath below the pouring temperature and then reheating it to the required temperature. By repeating this procedure, the minimum gas content can be obtained. The electric furnace facilitates degasifying in this manner. That occluded gases which are partly liberated in solidification, as well as non-metallic inclusions and segregation, are responsible for weakening the transverse structure is not yet perfectly established, but recent experiments seem to indicate this fact; therefore degasification is beneficial to the transverse properties of the steel and its importance should not be overlooked.
