Table of Contents
Pyrite oxidation has been studied extensively in relation to its role in sulfide mineral flotation. Although pyrite usually lacks economic value in polymetallic ores, it generally acts as an undesirable constituent which complicates the separation of valuable minerals. This mineral is also considered to be the main source of acid mine drainage. The formation of ferric ions and acids promote the dissolution of pyrite and worsens the quality of mine water.
Materials and Experimental Methods
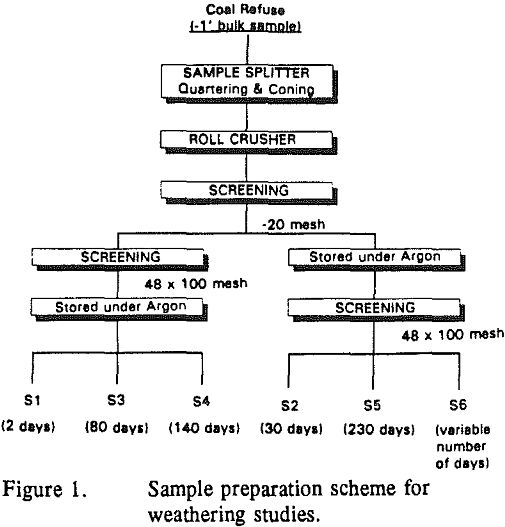
Oxidation of pyrite was studied using accelerated weathering tests in specially designed columns. The design of the column and the experimental procedure were selected so as to simulate weathering conditions in a vadose zone.
Electrochemical impedance spectroscopy was carried out to determine the effect of aging on the interfacial properties of pyrite. These measurements were made with an EG&G Princeton Applied Research impedance system consisting of a Model 5206 lock-in amplifier, a Model 173 potentiostat connected to an IBM-AT computer with a Model 276 interface. The impedance spectra were measured as a function of aging time in 0.1 M sodium borate solutions of pH 9.3. The electrode was kept at its open circuit potential (OCP) in nitrogenated solutions of 0.05 ppm O2 or less, throughout the aging period.
Results and Discussion
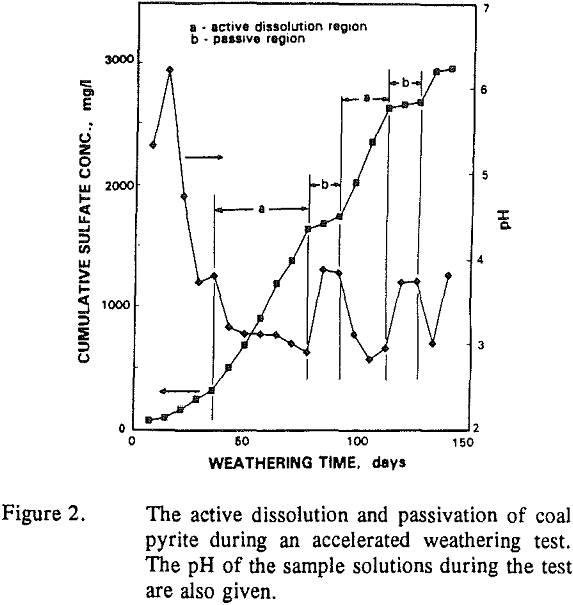
Even though pyrite oxidation has been widely studied and thoroughly reviewed, several aspects of the mechanisms involved remain unclear. Many products of oxidation have been identified but distribution of products at the surface and their influence on oxidation of pyrite is not known. The formation of surface films and their subsequent dissolution was observed in the kinetic studies performed as a part of this investigation.
- The duration of each active or passive period which is a measure of time for which pyrite dissolves or its dissolution is inhibited.
- The slope of the curve in each region which is a measure of the rate of oxidation.
- The transition from active to passive region or vice versa, which was gradual in some cases and sudden in others.
The pH of the solution sampled from the column increased from about 3 to 4 during the passive periods. The duration of the active or the passive periods was not constant, however. The passive periods generally ranged from 2 to 3 weeks, whereas the active periods lasted from 2 to 6 weeks. After 1 week of storage (dry oxidation) there was an initial passive period which lasted about 7 to 8 weeks after which pyrite became reactive, as can be seen in Figure 3a. For the sample stored in Argon, a second passive period was observed from 11 to 13 weeks of weathering after which the two samples were identical.
The amount of sulfate produced after 14 weeks of testing in accelerated weathering columns is given as a function of the time of storage. For the sake of comparison the results of oxidation in argon are also included. The results show that the effect of storage in air on the oxidation behavior of pyrite in weathering test columns was quite similar to that in argon. Although the surface properties of the pyrite stored in air and argon are not expected to be the same, the difference may not be identifiable after 14 weeks of weathering in the test column.
To determine the effect of aging on reactions of pyrite with various reagents, the effect of several dicarboxylic acid treatments on the weathering of aged pyrite was studied. These reagents were selected because of several reasons. First, carboxylic functional groups are present in humic acids produced by oxidation of coal. Thus an understanding of pyrite-reagent reactions will help delineate coal-pyrite interactions, if any. For the sample stored under argon for 1 month, the extent of oxidation of treated pyrite after 14 weeks of weathering followed the order:
Oxalic > succinic = glutaric > adipic > pimelic = suberic
The effect of sample storage time on the rate of oxidation of pyrite with and without the treatment of several reagents was investigated by accelerated weathering tests in laboratory columns. Coal pyrite stored under argon experienced changes in the surface properties. The changes occurred in several stages: a) loss of reactivity of the surface in the initial period probably due to formation of a S-rich layer; b) partial oxidation of iron sulfide to oxides or hydroxides, and c) formation of surface coatings of oxides or hydroxides after a prolonged time of storage. The variation in the surface character during the sample storage affected the oxidation behavior of coal pyrite upon weathering in the test column and its reactions with various reagents. These results imply that sample storage history must be considered if a kinetic test is to be used for determining acid generation potential of a pyrite bearing waste material.