The discovery that metallic gold is soluble in potassium cyanide came after studying the action of cyanides on plates of gold, and announced that they were slowly dissolved. Metallurgists pointed out that gold-leaf is dissolved by a dilute solution of the salt, and also showed that if the gold floats on the surface of the liquid, so that one side of the leaf is in contact with the air, while the other is bathed by the solvent, the action is much more rapid than if the metal is completely submerged. Elsner had previously furnished some evidence that the presence of oxygen is required for the solution of the gold.
On evaporating the solution, colourless octahedral crystals of auro-potassium cyanide, KAuCy2, are formed, which may be viewed as being a double cyanide, produced as follows:
4Au + 8KCy + O2 + 2H2O = 4KAuCy2 + 4KOH.
The equation for the solution of silver is similar. Theoretically, therefore, 130 parts by weight of KCy in the presence of eight parts of oxygen suffice for the solution of 196.8 parts of gold. This has been proved to be the case in all carefully conducted experiments. The amount of oxygen dissolved in liquids not specially prepared, to say nothing of that contained in a porous mass of pulverised ore, is consequently enough for the solution of great quantities of gold.
The chemical action in the dissolution of the gold is as follows:
2Au + 4KCy + 2H2O + O2 = 2KAuCy2 + 2KOH + H2O2
and subsequently
4KCy + H2O2 + 2Au = 2KAuCy2 + 2KOH
It is suggested the following in place of this:
- 6KCy + 2Au + O2 + 2H2O = KAuCy2 + KAuCy4 + 4KOH
- KAuCy4 + 2Au + 2KCy = 3KAuCy2
Evidence has been adduced that a substance is formed reacting like H2O2, but it does not follow that the actions represented by the equations given above are not limited to an insignificant part of the whole mass. Experiments have shown that gold in ores can be dissolved by potassium cyanide in the absence of oxygen, and found this to be the case if the crushed ore contains basic ferric sulphate, by which potassium ferricyanide is formed, the reactions being expressed thus:
- Fe2(SO4)3 + 12KCy = 3K2SO4 + K6Fe2Cy12
- Fe2(SO4)8 + 6KCy = Fe2(HO)6 + 2K2SO4 + 6HCy
Metallurgists found that gold leaf would not dissolve in a solution of potassium cyanide from which the air had been expelled by the passage of a current of hydrogen, and that the addition, under these circumstance, of either potassium dichromate, chromate, chlorate, perchlorate, nitrate, or nitrite or of ferric hydrate or bleaching powder, did not enable the gold to dissolve. The addition of pyrolusite gave a doubtful result, and lead dioxide caused very slow dissolution of the gold. On the other hand, gold dissolved slowly if chlorine, iodine dissolved in potassium iodide, or ferric chloride were added; rapidly, if bromine were added; and decidedly, if potassium ferricyanide or permanganate, sodium dioxide, hydrogen peroxide or barium dioxide were added. It is clear, therefore, that certain oxidisers are ineffective, and in practice, when air is not used, potassium permanganate, sodium dioxide, and bromine (Sulman-Teed process), have been mainly employed to assist in the dissolution of the gold. In general, artificial oxidation is not resorted to unless there is some reducing agent present in the ore or the water which absorbs the oxygen. It was formerly supposed that any oxygen coming in contact with the solution would be at once taken up by the cyanide, forming cyanates, but it is now well known that oxygen and cyanide remain side by side without rapid union between them taking place.
It has long been remarked that a small percentage of a soluble sulphide present in the cyanide solution greatly delays the dissolution of gold. Doubtless this is partly owing to the abstraction of oxygen from the solution by the sulphide, for gold sulphide is freely soluble in KCy, so that the surface of the metal is kept free from sulphide. Metallurgists, however, point out that silver sulphide is far less soluble than gold sulphide, and that if native gold containing 20 per cent, of silver is treated, a film almost insoluble in cyanide solutions may be formed. It is certain that some specimens of gold leaf dissolve with great difficulty if they have been previously dipped in sulphide solutions, or if traces of soluble sulphides or sulpliocyanides are present in the solution. The difficulty disappears if the sulphides are removed, either by being precipitated with lead salts, or by the action of certain oxidisers. The voltaic order of the metals in different solutions of cyanide of potassium is given in the following table:

The position of the metals in the tables denotes their relative tendency to dissolve under the given conditions. When two metals are in contact, the negative metal assists the positive metal to dissolve, and is partly protected by it from the attack of the solvent. Conversely a metal tends to precipitate from solution another metal that is negative to it, and to replace it in solution. It may be deduced that mercury is inapplicable as a precipitant for gold from solution, and that aluminium, copper, or zinc could be used to precipitate gold and silver from their solutions. Metallurgists proposed to use aluminium as a precipitant in presence of free alkali, when the cyanide is regenerated and alumina formed, thus:
6AuKCy2 + 6KOH + 2Al = 6Au + 12KCy + 3H2O + Al2O3
It is claimed that there is comparatively little waste of aluminium. The following table shows the actual amounts of metals (stated in grains per square inch of surface per hour) dissolved by a solution containing 1.86 per cent, of KCy:
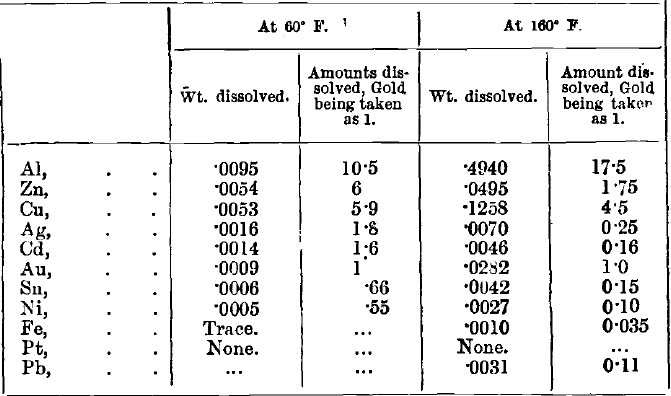
It will be observed that the rate of dissolution of gold is increased thirty-one times, and that the “ selective action ” in favour of it is also increased (except as regards aluminium) by the increase of temperature. It must be noted, however, that Dr. Gore’s table refers to metals in the form of sheets: it must also be observed that the solution of KCy employed was probably impure, containing much KOH. The aluminium would be dissolved by this, and the results affected generally. The position of zinc on the table is instructive. Dr. Gore has also shown that the rate of solution of gold is increased by 50 per cent, if it is placed in contact with iron, and that it is then five times more soluble than the iron.
Bagration states that the solution of metallic gold in cyanide of potassium is facilitated by a rising temperature. Little attention was paid to this point in the early days of the cyanide process, but in 1899 Loewy stated that experiments at the May Consolidated Gold Mine in 1893 yielded 10 to 14 per cent higher extraction when heat was applied. The ore treated was pyritic containing 50 to 60 per cent, of free gold, which was more affected by the heating of the solutions than the gold in the pyrites. Loewy considered that solutions should be heated above 35° C. G. A. Darling had previously pointed out that in the six “ cold ” months of the year in the Transvaal, from April to September, the average extraction during seven years, between 1891 and 1898, at the Robinson gold mine was 69.5 per cent., and in the “hot months,” October to March, the extraction was 72.2 per cent. Von Gernet at the same time stated that at the Worcester Cyanide Works during September, 1898, exhaust steam was passed through pipes to heat the solution, and that the residues had contained only 8 grains per ton although no comparative figures were available. On the other hand, it has been pointed out that in the “ hot months ” in South Africa there is more rainfall and the organic matter in the water is reduced, and the extraction might be improved from that cause, since the presence of organic matter reduces the free oxygen in the solutions.
J. S. Maclaurin found that the rate of dissolution of pure gold, in the form of plates, in potassium cyanide solution passes through a maximum when proceeding from dilute to concentrated solutions. The maximum is reached when the solution contains 0.25 per cent, of KCy. The solubility of gold is very slight in solutions containing less than 0.005 per cent., but increases rapidly as the strength rises to 0.01 per cent., when the rate of dissolution is ten times as great as in the 0.005 per cent, solution, and about half as great as that in the 0.25 per cent. The rate increases slowly as the strength rises to 0.25 per cent., and thereafter decreases much more slowly, until in 15 per cent, solutions the rate of dissolution is about equal to that in 0.01 per cent, solutions. Higher strengths show a gradual diminution in the rate of dissolution up to saturation point. Silver is also dissolved at a maximum rate in solutions containing 0.25 per cent, of cyanide, and the changes in the rate are similar to those noted above in the case of gold, the rates for silver being always about two-thirds of the corresponding rates for gold, or, roughly, in the same ratio as the atomic weights of the two metals. In both cases there is hardly any change in the rate of solubility as the strength rises from 0.1 to 0.25 per cent. From these results, it is seen that the most active solutions are now used in ordinary practice. It is remarkable that the solubility of oxygen in cyanide solutions continually decreases as the concentration increases, and it is to this fact that Maclaurin is disposed to attribute the variations in the rate of dissolution of the gold. The oxygen which unites with the cyanide, converting it into cyanate, is thereby made inactive, as the presence of cyanate of potassium has no effect on the rate of dissolution of the gold.
Christy, however, contends that the rate of dissolution of gold in cyanide depends on two factors, the electromotive force of gold in cyanide solutions, and that of the hydroxyl ions in the solution which may be assumed to be proportional to the solubility of oxygen, and consequently decreases as the transition is made from pure water through dilute cyanide solutions to strong ones. The E.M.F. of gold continually rises as the solutions become stronger, but is never large enough to enable gold to dissolve in the absence of oxygen. In pure water the E.M.F. of gold is —0.72 volt, in solutions containing about 0.043 per cent. KCy the E.M.F. becomes zero, and it then rises somewhat rapidly to +0.42 volt in a solution of 6.5 per cent. KCy, and thereafter more slowly to 0.468 in a solution containing 41.7 per cent. KCy. It follows from Nernst’s theory of solution pressure that solutions of gold in cyanide free from dissolved oxygen would remain undecomposed if they contained more than 0.043 per cent. KCy, but that gold would tend to precipitate spontaneously in the absence of oxygen in solutions containing less than 0.043 per cent. KCy. This requires experimental proof.
The presence of dissolved oxygen completely alters these results, and by direct experiments Christy found that solutions containing no more than 0.00065 per cent. KCy did not dissolve gold, and that for all practical purposes the cyanide of potassium solution ceases to act when its strength falls below 0.001 per cent. Above that strength, however, the solubility of gold increases rapidly.
In mill solutions, the free oxygen may be sometimes entirely removed and aeration is necessary. In long continued treatment of concentrates it may even be necessary to draw off the solution, partially dry the ore, and turn it over to expose it to the air before a fair percentage of the gold can be dissolved.
Owing to the use of the cyanide process, and owing also to the practice still adhered to of using potassium cyanide to clean the amalgamated plates, there is some danger of gold being dissolved in the stamp mill and lost. Care must be taken (when water is used over again in stamp mills) that the water is not contaminated by mixture with waste cyanide solutions. Von Gernet found that the water in the dams on the “Witwatersrand contained gold in amounts varying from traces up to 12 grains per ton.
Decomposition of Potassium Cyanide
Hydrocyanic acid is one of the weakest acids known, and is expelled from its salts by all mineral acids and many organic acids. Carbonic acid decomposes potassium cyanide in presence of water thus:
2KCy + CO2 + H2O = 2HCy + K2CO3
The smell of hydrocyanic acid, noticeable whenever KCy or its solutions are exposed to the air, is accounted for by this reaction.
In the presence of air, potassium cyanide takes up oxygen, and is converted first to cyanate and then to carbonate:
KCy + O = KCyO
2KCyO + 3H2O = K2CO3 + CO2 + 2NH3
These reactions are much more rapid if heat is applied. Strong solutions turn brown in the air. In dilute solutions, potassium cyanide suffers hydrolytic dissociation, and is partly changed into HCy and KOH. It results from this that the passage of a stream of any neutral gas such as nitrogen through the solution causes an evolution of hydrocyanic acid, while the solution becomes alkaline. The equation is:
KCy + H2O = HCy + KOH
The extent of the hydrolytic dissociation is 1.12 per cent, of the salt in a solution containing 0.65 per cent. KCy and 2.34 per cent, of the salt in a solution containing 0.16 per cent. KCy. In weaker solutions, the extent of the hydrolytic dissociation is more. If the solution is boiled with acids or alkalies, hydrolysis of the cyanide occurs rapidly, ammonia and formic acid being formed, thus:
KCy + 2H2O = NH3 + HCO2K
This equation does not represent the whole effect, as acetates and other organic substances are also formed. The reactions proceed slowly, even if the solution is cold and neither acids nor alkalies are present. It is obvious, from the observed facts of the decomposition of cyanide solutions mentioned above, that (1) the solution should be kept from contact with the air as far as possible, by having closely fitting lids to the storage vats, the zinc boxes, &c., and by transferring the solution from one to the other by means of iron pipes (not open launders); and (2) the solution must be kept free from acids, which can be effected by the addition of a little alkali.
