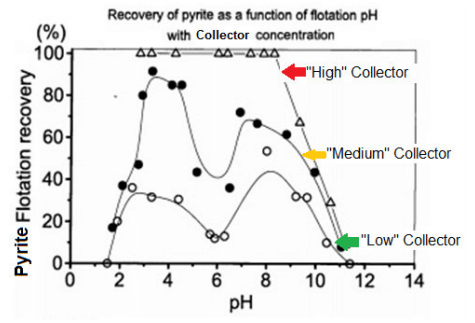
The curves below show how pH 5.5-6.5 is where conditions are optimum for iron depression. Shown on the previous chart but not these curves is this same pH 5.5-6.5 is also the optimum for Pb depression.
Note on the graph how iron recovery increases as collector dosage increases. Even at optimum pH 6, over dosage of collector will negate flotation selectivity and recover copper as well as iron/lead/zinc.
Also note how “letting go” of pH from 6 to 7 may have the similar effect on recovery as adding more collector and will less selectively recover more copper as well as iron/lead/zinc.
It is possible that as the mineralogy of some ores varies (Cu/Pb Ratio) and their grind liberation requirement become more demanding, better Cu Rougher Recovery results are obtained at pH 6.5 to 7.0.
As the Cu finds itself interlocked with more Pb and/or Fe, the Cu particles start to behave as Pb/Fe/Cu hybrids and begin to be rejected/depressed or at least “harder/slower” to float in the pH 6 environment. By “letting go” of the pH 6 and move it towards 7, it might then become beneficial to increase the recovery of poly-metallic mineral particles in the Cu rougher circuit before augmenting their liberation via the regrind circuit and truly reject the now liberated Pb and Fe via the Cu cleaners in a pH 6 environment.
In this situation, maximum regrind and optimum pH is applied as Pb and Zn loses to the Cu can climb rapidly if liberated Pb and Zn is allowed to float in a higher than optimum pH or over-collected environment.
Adequate Regrind Collector (5100)Dosage is essential as new fresh small Cu particles are created in the regrind. Until time and experience allows for something better, a ballpark starter rule-of-thumb is 1 cc/min for each 0.1% Cu in the feed. This will help Cu cleaner recovery.
Monitor the Cu/Pb/Zn head grades and adjust Frothers, Collectors like 5100, 3418A and Xanthate (CuSO4).