Table of Contents
We performed batch equilibrium contact experiments to examine the ability of activated coconut-shell carbon to adsorb various metal cyanide complexes. Tests were also conducted to determine the effect these cyanide complex impurities might have on the adsorption of gold by the activated carbon. Metal cyanide species included in the investigation were antimony, arsenic, cadmium, calcium, cobalt, gold, iron, mercury, nickel, silver, thallium, and zinc. Tests were made over a pH range of 6.4 to 12.5.
The carbon exhibited a preference for gold adsorption over other metals present in solution. In the absence of gold, the metal cyanide complexes were adsorbed; adsorption generally decreased with increasing pH. However, calcium and thallium adsorptions increased with increasing pH, while arsenic and iron were not adsorbed at any pH.
The use of activated carbon for the recovery of gold and silver from dilute caustic cyanide solutions has been well documented in recent years. Whether employed in carbon-in-pulp, carbon-in-leach, or loading-column processes, activated carbon has amply demonstrated its ability as an efficient extractor of precious metals from cyanide leach solutions.
With the discovery and treatment of disseminated gold deposits in the Western United States and in the Republic of South Africa, many complex ore deposits are being leached with cyanide. As a result, cyanide complexes of antimony, arsenic, cobalt, copper, iron, nickel, thallium, and zinc may appear in the leach solution that is contacted with activated carbon. Those metals contained in the pregnant leach liquor may or may not follow the precious metals through the carbon circuit. Few investigations have been conducted to determine the adsorption behavior of auxiliary metals onto activated carbon.
The U.S. Bureau of Mines main objectives of this study were to determine the effect of pH on the equilibrium loading of various metal cyanide complexes onto activated carbon and the effect of accessory metals on gold adsorption. Because both H+ and OH- tend to be adsorbed onto carbon, the adsorption of other species is influenced to a considerable degree by solution pH. Several investigators have observed that gold-loading onto activated carbon is strongly dependent on solution pH, with the amount of gold adsorbed in the pH range 4 to 7 being about twice that adsorbed in the pH range 8 to 11.
Davidson explained that the degree to which various gold cyanide complexes are adsorbed onto activated carbon is dependent on the complementary cations present, decreasing in adsorption strength in the order Ca²+ > Mg²+ > H+ > Li+ > Na+ > K+. He also showed that the adsorption capacity of activated carbon for gold in the presence of 2,000 ppm Na decreased with increasing pH. The gold adsorption rate was increased considerably in the presence of sodium or calcium when the pH of the solution was lowered.
Boehme and Potter determined the effects of copper, ferrocyanide, silver, and thiocyanate on the rate of gold loading. As copper concentration increased, the gold-loading rate decreased. Silver had an adverse effect on the loading rate of gold in ratios of silver to gold of 1:1 and 2:1. A cyanide concentration above 2 g/L also inhibited the loading rate. Ferrocyanide had no effect on loading rate, while thiocyanate was not as detrimental as copper or silver, but a decrease in loading rate was observed.
Fleming and Nicol showed that loading competition between gold and various copper cyanide complexes was dependent on pH and free cyanide concentration. Cho and Pitt demonstrated that sodium ions increased silver loading, while cyanide ions had the opposite effect.
Activated carbon is known to adsorb organic solvents that affect the rate of loading rather than the equilibrium- loading capacity of gold. Activated carbon will load small quantities of calcium carbonate, cyanide, hydroxide, and iron sulfide, which poisons the carbon, resulting in a loss of gold capacity. In addition, cobalt, copper, iron, and nickel also adsorb onto carbon. Copper, and to a lesser extent nickel, may or may not load more strongly than gold, depending on the chemical conditions in the solution. The chemistry of copper cyanide has been extensively investigated and thus was not included in this study. These metals reduce gold loading by competing with the gold for adsorption sites on the carbon.
There are currently a variety of accepted theories for adsorption mechanisms on activated carbon. There is much confusion and little agreement of adsorption mechanisms, particularly gold-adsorption mechanisms. This report, therefore, does not attempt to explain the results obtained but to simply report them. The theories fall into four general categories as follows;
- reduction of Au(CN)2- to gold metal,
- adsorption of Ca(Au(CN)2)2 ion pairs,
- gold cyanide precipitation from degradation of Au(CN)2-, and
- double-layer adsorption of Au(CN)2- and cations onto a charged surface, with partial reduction of Au(CN)2- to cluster-type species.
As part of its program to assure an adequate supply of metals and minerals from domestic sources, the Bureau investigated the effect that metal impurities, present in cyanide leach solutions, have on the adsorption of gold by activated carbon. The metals used in the survey were typically associated with complex ore deposits of the Western United States and included antimony, arsenic, cadmium, calcium, cobalt, iron, mercury, nickel, silver, thallium, and zinc.
Experimental Procedure
Preparation of Activated Carbon
Coconut-shell activated carbon, minus 6 plus 16 mesh, was activated by heating to 600° C. It was then stored in deionized water in order to maintain activity. For each experiment, the parent carbon was sampled using a splitter to provide homogeneous fractions of 2.4 mL wet-settled carbon. A settled volume of 2.4 mL corresponds to 1.0 g of dry carbon.
Preparation of Contact Solutions
Contact solutions were prepared by dissolving reagent-grade metal compounds in deionized water to give a metal concentration of 150 ppm. Sodium hydroxide was added to maintain a pH above 10. Sodium cyanide was then added to give an initial free cyanide concentration of 0.5 g/L.
The effect of the cyanide complex impurities on carbon adsorption of gold was determined by adding sodium gold cyanide to the synthetic feed solution, producing a gold concentration of 150 ppm. This concentration is significantly higher than metal concentrations normally encountered in most gold plant solutions; however, the results are indicative of the loading phenomena.
Following the removal of a solution head sample for metal analysis by atomic absorption, the appropriate radioactive tracers for the cyanide complex impurity and gold were added to the solution. A minimum amount of tracer was added to the head solution, which produced an adequate statistical measurement on the gamma or beta detectors. The reagents and radioactive tracers that were used are listed in table 1.
Contact Test Procedure
The experimental procedure for determining equilibrium loading onto the activated carbon was as follows:
- Two hundred milliliters of the radioactive feed solution was pipetted into a 250-mL plastic sample bottle;
- The pH of each solution sample was adjusted to a specific value between 6.5 and 12.5 using either sodium hydroxide or hydrogen chloride;
- The wet-settled carbon samples were decanted, and a carbon sample was added to each bottle;
- Contact equilibrium loading was achieved by rolling the bottles for 50 h;
- Following contact, the equilibrium pH was measured;
- Metal adsorption was determined by counting the radiation emitted from solution after contact and comparing it with the amount of radiation emitted by the feed solution.
Results and Discussion
Effect of pH on Metal Cyanide Complex Loading
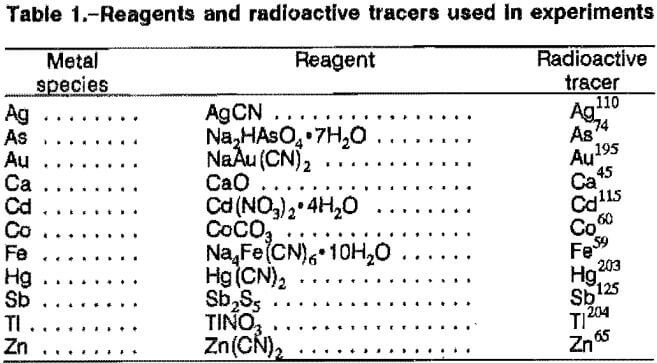
Initial experiments were performed to determine how the individual metal cyanide complexes were adsorbed by activated carbon. The results of this investigation indicated that the contaminant metals surveyed can be divided into five categories according to their loading characteristics: (1) mercury and silver, (2) antimony and nickel, (3) cadmium, cobalt, and zinc, (4) arsenic and iron, and (5) calcium and thallium.
The adsorption curves for mercury and silver were very similar to the baseline adsorption curve produced by gold. Figure 1A shows that maximum metal extraction occurred below pH 8 and decreased with increasing pH. Maximum extractions obtained were 97, 96, and 80 pct for mercury, gold, and silver, respectively. As the solution pH increased to 12, the respective extractions decreased to 38, 61, and 43 pct.
Antimony and nickel, shown in figure 1B, also exhibited a trend toward decreasing metal extraction as pH increased. The maximum extractions of 60 pct Sb and 42 pct Ni, however, were considerably lower than those for the previous group of metals. The decrease for antimony was delayed by an adsorption plateau of 60 pct between pH 9.7 and 11.2. Adsorption plunged to 1 pct at pH 11.6, then rapidly increased to 12 pct at pH 11.9. The sudden decrease may easily be attributed to the decrease in antimony solubility and subsequent metal precipitation. Nickel adsorption plateaued at 42 pct between pH 8.9 and 10.7, then reached a minimum of 35 pct at pH 11.5.
Cadmium, cobalt, and zinc, shown in figure 1C, also exhibited declining adsorptions with pH increase. However, unlike antimony and nickel, the curves exhibited a rapid initial decrease with increasing pH. The maximum extractions were 29.9 pct Cd, 27.3 pct Co, and 32.9 pct Zn, achieved at the lowest pH levels tested.
The loading behavior of calcium and thallium, shown in figure 1D, was opposite to the general loading behavior observed for the other metals. Metal adsorption was found to increase as pH increased. The adsorption of calcium increased from a minimum of 35 pct at pH 6.4 to 56 pct at pH 11.5. Previous investigators speculated that calcium precipitated onto the carbon above pH 7.5, which would account for the decrease in calcium concentration in solution with increasing pH. Carbon adsorption of thallium increased from 14 pct at pH 6.8 to 55 pct at pH 12.5.
The curve for iron adsorption, also illustrated in figure 1D, showed an increase in adsorption with increasing pH, up to approximately pH 9, where it leveled off at a maximum of 10 pct. Arsenic did not adsorb at any pH.
Effect of Metal Cyanide Complexes on Gold Loading
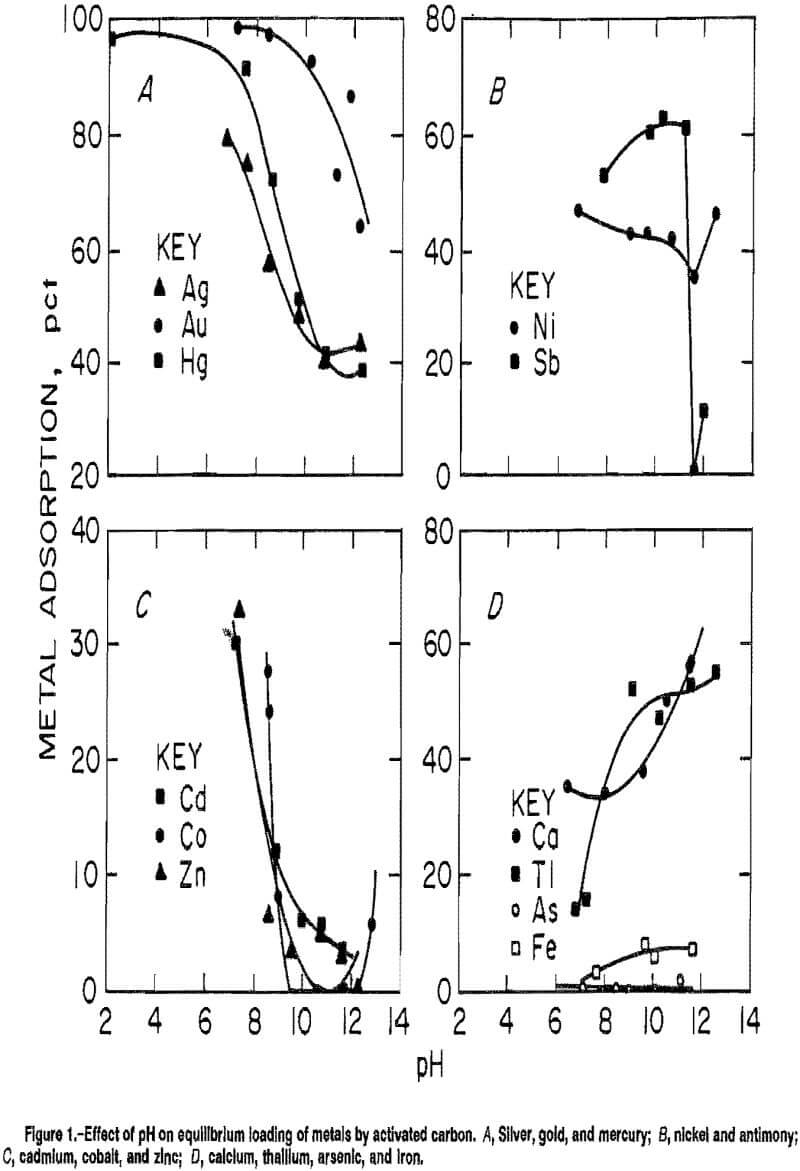
One of the main purposes of this investigation was to determine what effect the presence of metal cyanide complexes in the solution may have on the adsorption of gold by activated carbon. Experiments were performed with solutions that contained 150 ppm Au in addition to the individual metal complexes discussed in the previous section. The results given in figure 2 show gold-loading curves in the presence and absence of accessory metals.
The gold-loading curves generally indicate a pH independence between pH 6 and 12 with an adsorption of 98 pct when an accessory metal is also present at the same concentration as the gold. The improved gold loading that was observed when other accessory metals were present may be attributed to the increase in ionic strength of the solution. When the zinc complex was present, however, the adsorption was lowered to 88 pct with no pH influence.
An exception to the pH effect was observed when thallium was present. Of all the accessory metals studied, thallium was the only one that produced a gold-loading curve similar to the curve for the accessory metal alone in solution. In this case, gold adsorption increased with increasing pH, from 64 pct at pH 6.8 to a maximum of 78 pct at pH 12.3. From pH 6.8 to 11.3, gold adsorption was lower in the presence of thallium than in its absence.
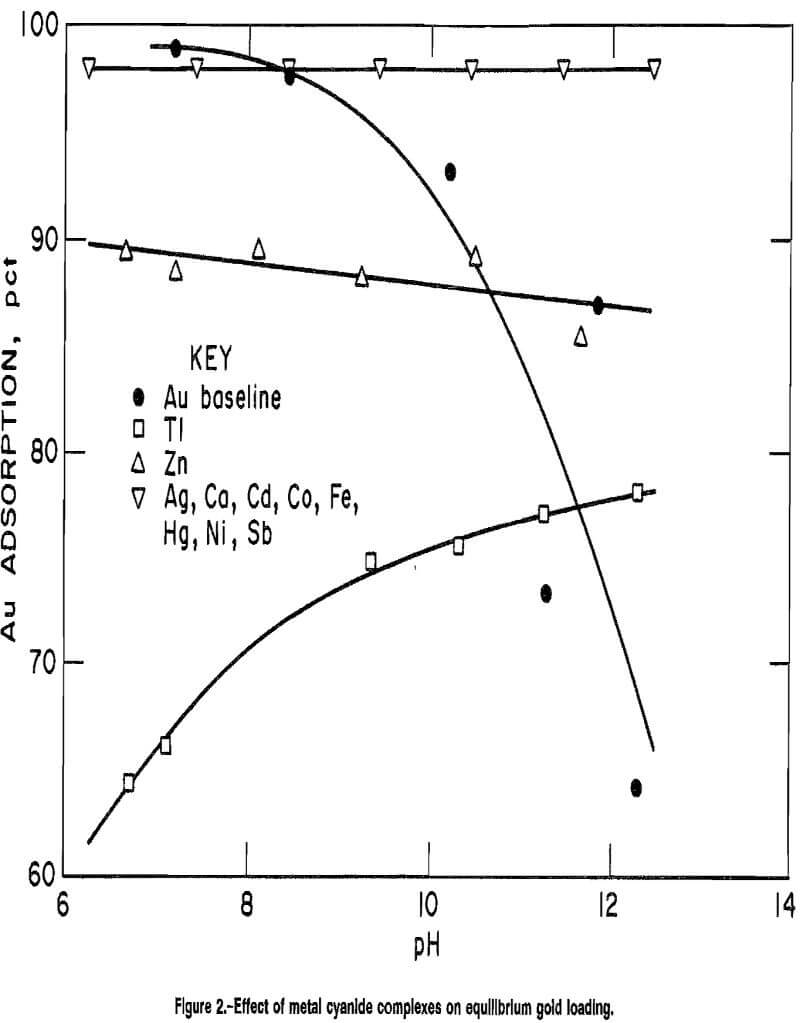
Effect of Gold on Metal Cyanide Complex Loading
Figure 3 illustrates the adsorption curves for each of the metals examined in the previous section in the presence of gold. Generally, the adsorption behavior of the accessory metal was similar to its behavior without the presence of gold at pH 9 and below. The exceptions were antimony and thallium, which exhibited greater adsorption when gold was also present.
Abrupt increases in metal cyanide adsorption, in the pH range 9.5 to 12, were observed for cadmium, cobalt, mercury, nickel, silver, and zinc when gold was present. This behavior may have been due to the increased ionic strength of the solution as excess sodium or hydroxide ions were added for pH control in these gold-containing solutions.
Conclusions
As a result of this investigation, the following conclusions were reached relating to the loading behavior of gold and other metals onto activated carbon from cyanide solution:
- The adsorption of gold was enhanced above pH 8 by the presence of antimony, cadmium, calcium, cobalt, iron, mercury, nickel, and silver. Gold adsorption with these metals in solution was independent of pH and averaged 98 pct. The presence of zinc also caused pH independence but with a lower adsorption of 88 pct.
- Thallium in solution with gold produced a gold-loading curve that reflected the loading curve of thallium when alone in solution. Gold adsorption increased with increasing pH, from 64 pct at pH 6.8 to a maximum of 78 pct at pH 12.3.
- Calcium and thallium were the only metal cyanide complexes examined for which adsorption onto activated carbon increased as pH increased. Antimony, cadmium, cobalt, gold, mercury, nickel, silver, and zinc exhibited decreasing adsorption as pH increased. Arsenic and iron adsorbed less than 10 pct.
- The presence of gold cyanide reduced the adsorption of antimony, calcium, cobalt, iron, mercury, and silver by activated carbon. However, adsorption of cadmium, nickel, thallium, and zinc increased in the presence of gold.
