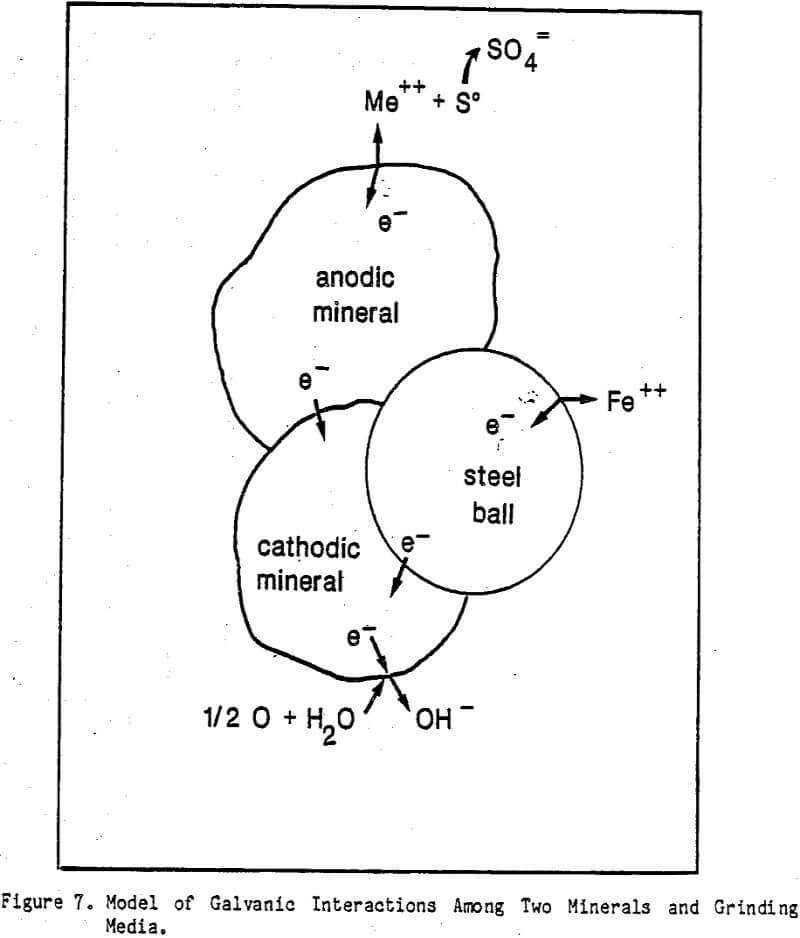
The flotation behaviors of pyrite and pyrrhotite in a Hallimond tube were correlated with the galvanic role they developed in two- and three-electrode combinations with mild steel under non-abrasive conditions. These correlations substantiated the hypothesis that a coating of iron hydroxides, originating from galvanic interactions, lowered the flotation responses of sulfide minerals. However, interactions between metal debris particles, generated by the abrasive action of grinding on the steel media, and minerals had overwhelming effect on the flotation responses of the minerals over the iron oxide film formation. These mineral-metal debris interactions became particularly significant in the case of pyrrhotite where magnetic forces are involved. As a consequence, the flotation responses of ball mill ground minerals became strongly dependent upon the hardnesses and electrochemical activities of the grinding media and also upon the partial pressure of oxygen in a ball mill.
 |
 |
Experimental
Quartzite from Minnesota (3.19% Al2O3, and 0.56% Fe), pyrrhotite from Canada (57.52% Fe, 33.35% S and 0.31% SiO2) and pyrite from Huanzala, Peru (45.84% Fe, 52.7% S, and 0.8% SiO2) were used in the flotation and electrochemical tests. Even though mild steel (AISI 1020, 02% C, hardness 127 Hv), a poor abrasion and corrosion resistant material, is not used in grinding practice, it was selected for the purpose of this study to magnify the effect of abraded steel debris on the electrochemical behaviors of sulfide minerals.
For micro-flotation tests, pyrrhotite and pyrite particles of 74/44 µm (200/325 mesh) sizes were used. The sample preparation method for pyrrhotite was described elsewhere. In order to obtain reproducible results in the micro-flotation tests, the pyrrhotite sample was cleaned with a sulfuric acid solution of pH 2.2 and washed several times with distilled water prior to their use. The pyrite sample was prepared by grinding in an agate mortar and pestle and by screening out the 74/44 µm fraction, and used immediately without any further treatment. The pyrite and pyrrhotite samples used for batch flotation tests in a Denver laboratory flotation cell were crushed through 10 mesh. The crushed pyrrhotite sample was then concentrated with a hand magnet and stored in an air-tight glass bottle.
To investigate the electrochemical characteristics of grinding media materials and minerals used in this study, electrodes of pyrrhotite, pyrite and mild steel were prepared by mounting the samples in lucite tubing of 0.625 cm I.D. with a 3M Scotch-Weld structural adhesive, so that about 0.16 cm² of their surfaces were exposed to the solution. The electrodes were cleaned on a metallurgical polishing wheel with alumina powder (0.5 µm) as the abrasive material. The electrical contact with the external circuit was made by placing a few mercury drops to connect with a copper wire.
A schematic diagram of the experimental setup is shown in Figure 1. It was designed for galvanic current measurements on a three electrode system. It consisted of a zero resistance ammeter (G) and the electrodes E1, E2 and E3. A switch permitted the rotation of the ammeter connection with the electrodes in such a way that the corrosion current of each electrode could be separately and alternately monitored. The measurements were made with an EG & G Model 350A Corrosion Measurement Console. A saturated calomel electrode (SCE) was used as the reference electrode via a salt bridge containing a saturated solution of ammonium nitrate.
For micro-flotation tests, a modified Hallimond tube was used to investigate the effect of galvanic interactions on the floatabilities of the minerals. The effect of galvanic contact was examined by placing one gram of pyrrhotite and/or the same amount of pyrite in a mild steel crucible along with 50 mL of distilled water. The pulps were left exposed to the air for 20, 60 and 120 minutes. When both minerals were contacted simultaneously with mild steel, their floatabilities were individually evaluated by magnetically separating the pyrrhotite from the pyrite with a hand magnet.
The samples, after decantation, were transferred to a 100 mL Pyrex flask containing a solution of a collector, conditioned by tumbling for 5 minutes and floated for 5 minutes in the Hallimond tube using nitrogen at a flow rate of 50 mL per minute.
The effect of a pyrite-pyrrhotite-mild steel contact under abrasion cannot be studied in the micro-flotation test setup. Accordingly, batch flotation tests were performed on freshly-ground artificial mixtures of quartzite with either 55 pyrite and/or 5% pyrrhotite in a Denver laboratory flotation cell. The mixtures in the amount of 600 g were ground at 60% solids to minus 100 mesh in a 203 mm (8-inch) porcelain mill containing 126 mild steel balls of nominally 25.1 mm (1 inch) in diameter under a nitrogen or an oxygen atmosphere. Either potassium ethyl xanthate (KEX) or potassium butyl xanthate (KBX) was used as a collector and the flotation tests were carried out for 3 minutes using nitrogen for aeration in order to prevent the oxidation of abraded steel debris during flotation.
Micro-flotation tests
Initially, two series of tests were carried out with freshly ground mineral samples to determine the collector concentrations needed to attain the full recoveries of pyrrhotite and pyrite. The recovery of pyrrhotite reached 92% at 5 x 10 -4 mol/L KBX and 89% for pyrite at 4 x 10 -4 mol/L KEX. Further increase in the collector additions did not improve the recoveries of the minerals.
Two types of tests were performed:
- Flotation of pyrrhotite and pyrite samples after contacting them individually with mild steel.
- Flotation of pyrite and pyrrhotite samples after contacting them together with mild steel.
The results obtained are depicted as a function of time in Figure 3. The figure shows that the adverse effect on the flotation recoveries of contacting pyrrhotite or pyrite with mild steel individually is partially reversed by contacting them together with mild steel.
The above flotation results may be correlated with the galvanic current data for the two- and three-electrode systems, as presented in Table 1. From this correlation, it is apparent that the higher the rate of the cathodic reaction at a mineral surface, the stronger the adverse effect on its flotation recovery. Such an observation would be consistent with the hypothesis and substantiated in subsequent investigations, which proposed that the formation of an iron oxide coating on mineral surfaces is responsible for the adverse effect of galvanic interactions on the flotation recoveries of the sulfide minerals.

