Table of Contents
For maximum blast furnace productivity it is generally believed that the iron oxide pellet should contain as many of the ingredients of the burden as possible. As a result over the past 10 years considerable research effort has been directed towards the development: of self-fluxed iron oxide pellets. Calcitic limestone has been used as the major flux in conjunction with bentonite binder. Self-fluxed pellets with basicities ranging from 0.5 to 1.5 have been successfully produced in large tonnages in both the traveling grate and grate-kiln systems at temperatures 50 to 100 degrees lower than regular (acid) pellets. In spite of the lower firing temperatures their mechanical properties, i.e., compressive strength and abrasive resistance, have proven to be equal or superior to regular pellets. Information on the metallurgical behavior of self-fluxed pellets is less conclusive and often contradictory. In general, however, much of the published literature agrees that under reducing conditions simulating those existing in the upper stack of the blast furnace these pellets often show greater swelling, less resistance to softening, and a higher degree of disintegration than regular pellets.
How self-fluxed pellets will respond in a commercial blast furnace and what economic rewards can be expected remains to be seen. Research in this area has been limited and the few available results, although encouraging, are in conclusive. The reportedly high disintegration of some self-fluxed pellets under reducing conditions has undoubtedly restrained testing of these pellets in commercial blast furnaces.
The Twin Cities Metallurgy Research Center, as part of its broad research program on the utilization of low-grade iron ores, is currently evaluating the effectiveness of certain basic additives on the mechanical and metallurgical properties of green and fired pellets. This paper describes the preliminary bench scale and pilot plant research with dolomitic ‘burnt’ lime as the basic additive for preparation of self-fluxed pellets from a typical magnetic taconite concentrate. Dolomitic ‘burnt’ lime, (CaO.MgO) was used in preference to dolomitic limestone, CaMg(CO3)2, and dolomitic hydrated lime since earlier studies at the Center showed that it gave higher green, dried and fired pellet strengths when measured at equivalent basicity levels. In contrast to the reportedly high tendency for some self-fluxed pallets made from calcitic limestone to break down under conditions simulating their descent through the blast furnace (Linder test), the reduction disintegration resistance of the dolomitic lime pellets produced in the pilot plant was greater than for the regular pellets.
Equipment and Procedure
Materials
A high-grade magnetic taconite concentrate (67% Fe) obtained from a commercial facility in northern Minnesota was used in the investigation (table 1). The concentrate contained 92.5 percent magnetite and 5.5 percent silica and had a sire consist of 92 percent minus 325 mesh. The material was typical of the magnetic concentrates currently being produced in the Lake Superior District.

An industrial-grade, pulverized dolomitic ‘burnt’ line (Ca0,Mg0) was used as the flux additive in both the bench scale and the pilot plant tests. The lime (as received) was extremely fine (-97%, minus 325 mesh) and contained less than 1 percent impurities (table 2).
Bench Scale Studies
Self-fluxed pellets were made under controlled laboratory conditions to determine the effect of lime concentration on the green, dried, and fired pellet strengths. The iron oxide-lime mixtures (without bentonite) blended in various weight ratios to obtain fired pellet basicities ranging from 0.5 to 3.0, were formed into minus 5/8 plus ½ inch diameter balls in a 15-½ inch diameter balling drum. The green pellets were indurated in an electric muffle furnace at temperatures up to 1,300° C in an oxidizing atmosphere. The heat hardening procedure consisted of placing 60 predried green pellets in the furnace set at 450° C. The furnace was brought to the desired test temperature, held there for 30 minutes and allowed to cool to 450° C before the pellets were removed.
Pilot Plant Studies
Regular and self-fluxed iron oxide pellets were produced on a continuous basis in a modified traveling grate machine. The objectives of the pilot plant tests were to determine the pelletizing characteristics of the basic pellets under direct firing conditions and to produce a sufficient quantity for evaluation tests. For this study, self-fluxed pellets (B = 0.81 and B = 1.55) made with dolomitic lime (without bentonite) were compared with regular pellets (B = 0.16) containing the bentonite binder. The pellet mix for regular and self-fluxed (pilot plant) pellets is listed in table 3.
Flowscheme and Equipment
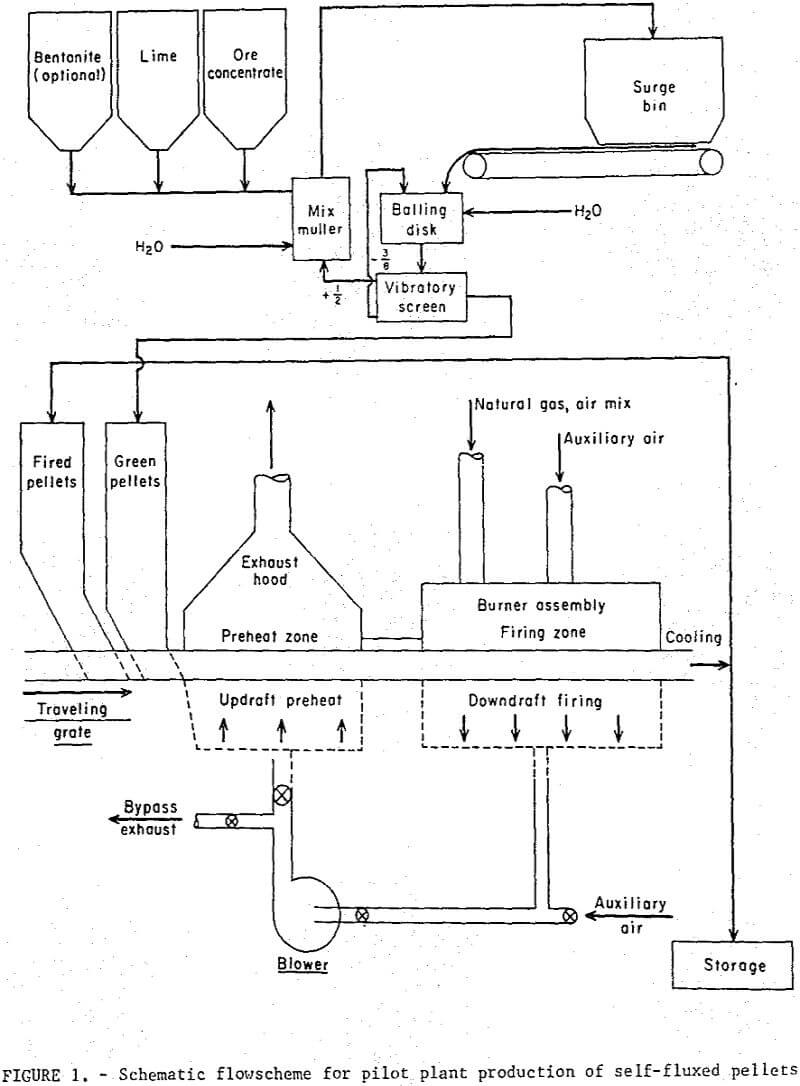
A schematic diagram of the overall pilot plant circuit is depicted in figure 1. The magnetite concentrate and additives were blended in a mix muller prior to formation into minus ½ plus 3/8 inch balls in a 3 foot diameter balling disk. No major problems were encountered with either mixing or balling the magnetic concentrate containing the lime additive. Reaction of the lime with water reduced the moisture of the wet concentrate during the mixing operation and additional water was required to maintain good balling conditions.
A 12-inch wide by 10-foot long traveling grate sintering machine was used for induration. The original machine was modified to incorporate an updraft preheating section which utilized the hot recycled combustion gases from the downdraft firing section to dry the wet pellets. The burner assembly consisted of eight refractory screen burners mounted in a high alumina castable refractory. The burners were located along the full length of the 4-foot firing section and were designed to allow adjustable burner-to-bed distances.
The air-natural gas mixture was supplied by two Venturi-type flowmeters with separate manifolds, each feeding four individually controlled burners. The desired air gas ratio was monitored automatically and provisions were made for drawing secondary air into the various heating zones, A Pitot-static tube was used to measure recycled gas flow rates. Numerous sheathed thermocouples were positioned above the pellet bed and below the grate bars at a distance of 1 inch throughout the grate system. The interior bed temperatures in the firing zone were measured with a thermocouple inserted through the side of one of the moving pallets. An optical pyrometer was used to measure the bed surface temperature under the burners.
Operation
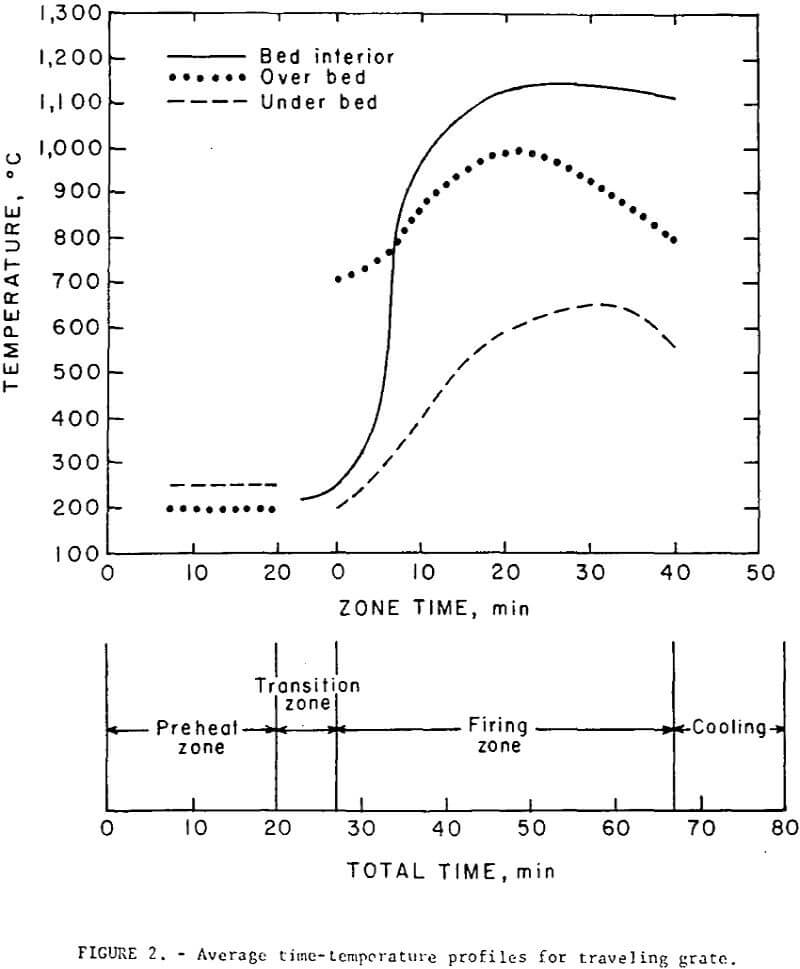
Both the regular and self-fluxed pellets were produced under similar operating conditions. Green and fired (hearth) pellets were fed onto the grate from separate stationary hoppers mounted on the feed-end. A maximum pellet depth of 5 inches was used in all tests; this included a 2 inch layer of cold, fired pellets (hearth layer) and a 3 inch layer of green pellets. The hearth layer was required to prevent overheating of the cast iron grate bars and excessive heat losses. A neutral flame was maintained at the burners; secondary air, drawn through the firing zone from openings in and around the burner assembly amounted to about 300 percent excess air. Gas flow rate (including secondary air) from the firing zone was 85 scfm/ft² and contained approximately 15 percent oxygen; the gas temperature of 477° C was quenched to 230° C by opening auxiliary air-intake valves before recycling to the preheat section. Most of the drying of the green pellets occurred in the hopper at 200° to 220° C. The pellets were in the preheating section of the grate for about 20 minutes. No thermal decrepitation or spalling was observed with either the bentonite or lime pellets during the drying and preheating cycles. The average time-temperature profiles maintained in all tests are shown in figure 2; all temperatures were measured at the center line of the bed. The pellets were maintained at a mean temperature of 1,150±25° C for a period of about 30 minutes. It had been thought that, particularly with the higher basicity pellets (B = 1.55), the direct flame impingement might cause fusion of the surface pellets thereby decreasing the bed permeability, but, little, if any, fusion occurred even at points directly under the burners where the temperatures exceeded 1,300° C.
The discharge product was sampled continuously through the entire 3 inch depth but only along the center line of the grate. Pellets at the edges were underfired because of the heat loss through the side walls and were not included in the test sample. Operating data for the pilot plant tests are listed in table 4.

Results and Discussion
Mechanical Properties of Green and Fired Pellets
Green Pellet Quality
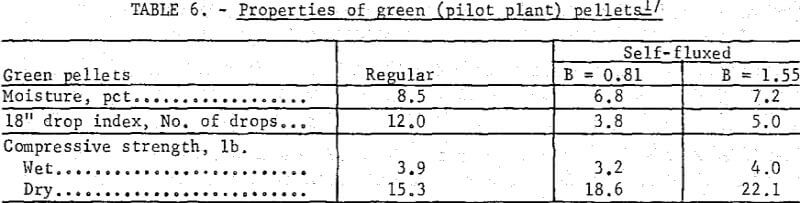
Similar trends were noted in the strength properties of bentonite and lime pellets produced on the bench scale (table 5) and pilot plant levels (table 6). The bentonite pellets had a higher moisture content, drop index and plasticity. The wet compressive strengths of the two pellet types were comparable although higher dry strengths were obtained with increasing lime additions. The lower plasticity and high dry strengths of the lime pellets facilitated their feeding to the grate machine from the stationary hopper. For production of self-fluxed pellets with a basicity of 1.0 to 1.5 from concentrate containing 4 to 8 percent SiO2 a lime addition of about 6 to 12 percent would be required. With this amount of lime it is highly probable that a bentonite binder would not be required to maintain adequate pellet quality.
Fired Pellet Quality
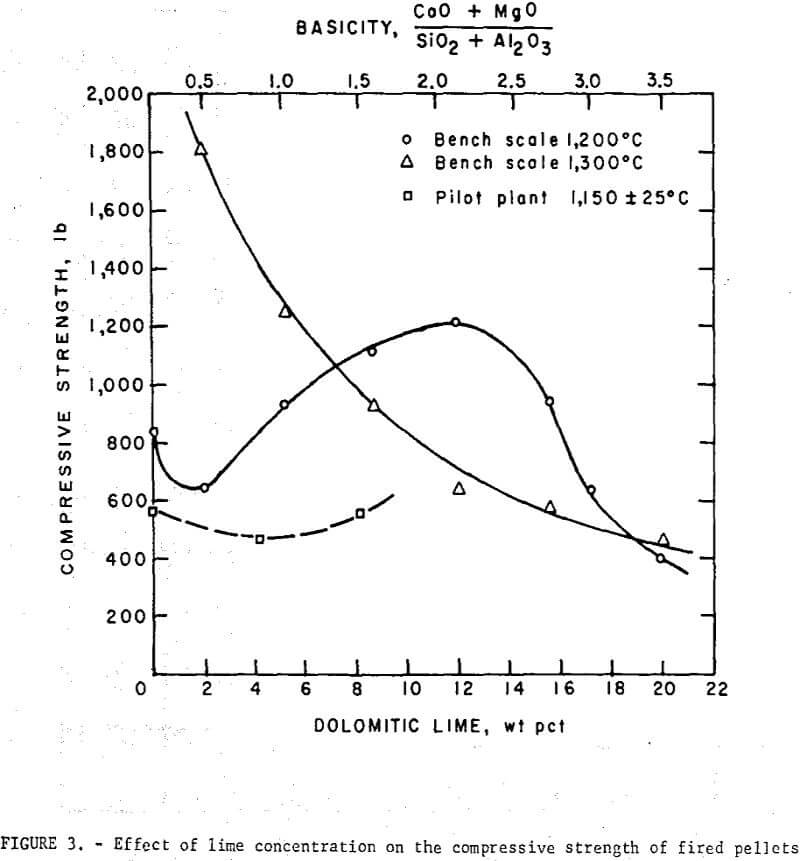
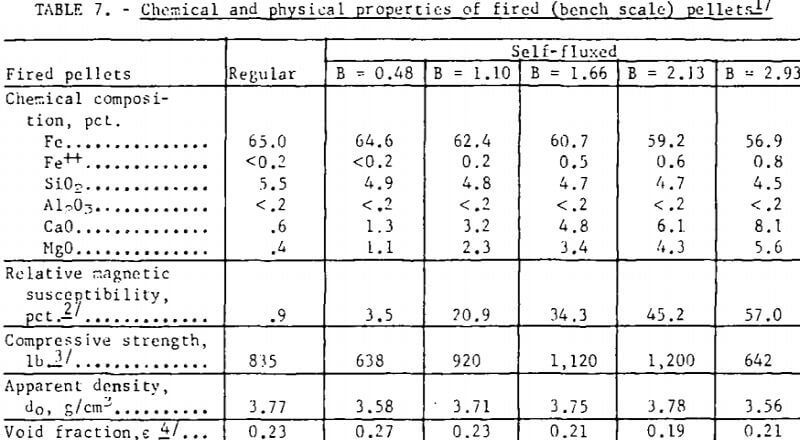
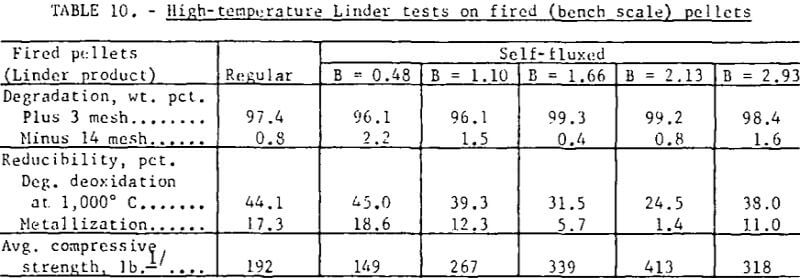
Bench scale tests revealed some interesting trends in the fired strength patterns of self-fluxed pellets. It should be noted however that the pellet strengths will generally be higher than would be obtained under pilot plant conditions since it was not possible to simulate rapid preheating, firing, and cooling cycles in the muffle furnace. Typical patterns for pellets fired at 1,200° and 1,300° C are depicted in figure 3. At 1,200° C a minimum compressive strength was obtained with lime additions of about 2 percent (B = 0.5) reaching a maximum with 11 percent lime (B = 2.0) and decreasing, again at higher basicities. Similar patterns were observed at 1,000° and 1,100° C. At 1,300° C increasing lime additions resulted in a continuous deterioration in room temperature pellet strength. Temperatures ranging from 1,200° to 1,250° C appear to be the most favorable for production of self-fluxed pellets with basicities above 1.0. It was also found that the lime concentration influenced the fired pellet porosity (table 7). Minimum pellet porosity occurred in the basicity range of 1.5 to 2.0 (8 to 11 percent lime additions). An inverse relation between pellet porosity and compressive strength was noted over the basicity range studied.
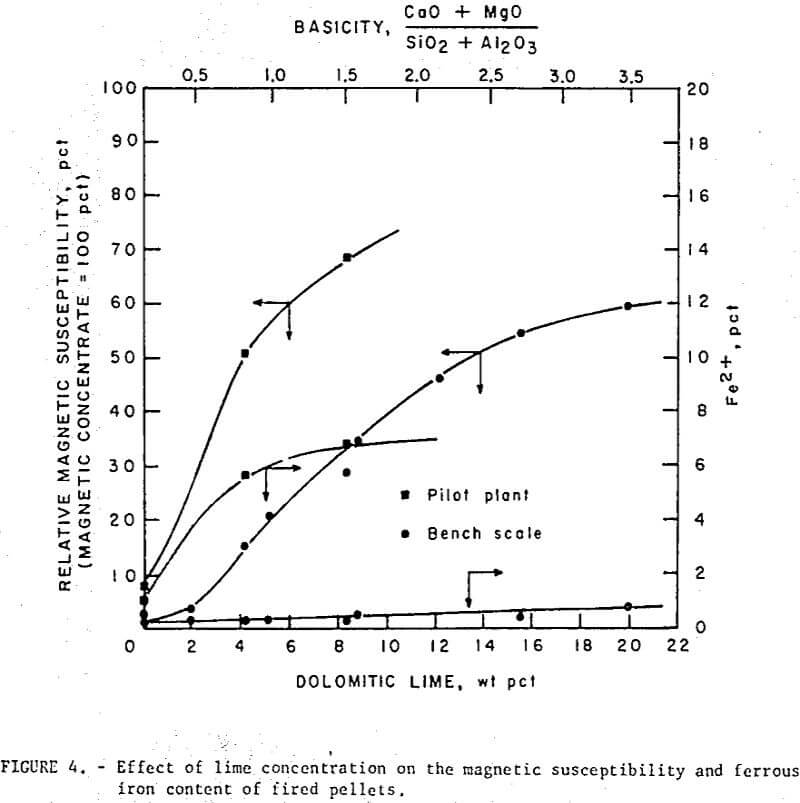
Unlike regular pellets (or self- fluxed pellets made with calcitic lime) the self-fluxed pellets with dolomitic lime were magnetic after induration (fig. 4). This high magnetic susceptibility, was attributed to the formation of magnesioferrite, MgO·Fe2O3), (or some solid solution of the composition (FeO,Mg0)·Fe2O3) , a ferrimagnetic species with a spinel structure similar to that of magnetite. As noted (fig. 4), the self-fluxed pellets produced in the pilot plant increased in ferrous (Fe++) iron with increasing basicity. The bench scale studies, however, showed less than 1 percent Fe++ in self-fluxed pellets after induration at 1,200° C. The low Fe++ in the latter pellets was due to the slow furnace cooling and greater opportunity for reoxidation; ferrous iron was observed in similar pellets that had been quenched at 1,200° C. Dissociation studies made on Fe2O3-MgFe2O4 mixtures in air indicated that Fe++ begins to form for all compositions at temperatures above 800° C which is well below the reported dissociation temperature of both Fe2O3 1,392° C) and MgFe2O4 (-1,415° C). For the Fe2O3·MgFe2O4- rich composition (self-fluxed pellet range) it has been postulated that the equilibrium reaction:
3Fe2O3 ↔ 2Fe3O4 + ½ O2…………………………………………………..(1)
is forced to the right by lowering of the free energy of the Fe3O4 through solution in MgFe2O4 as the latter has the same spinel crystal structure. The possibility that the presence of ferrous iron, in the self’-fluxed pellet was the result of incomplete pellet oxidation appeared unlikely since regular pellets treated under similar conditions were low in ferrous iron.
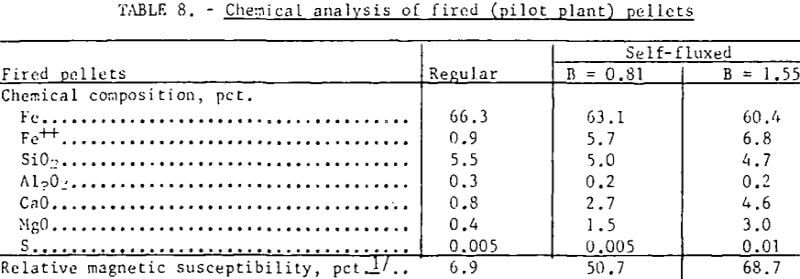
Analysis of regular and self-fluxed (pilot plant) pellets is listed in table 8. The iron content of the fired pellets was lowered by approximately 0.7 percent for each percent lime addition to the pellet mix. As expected, because of the low sulfur in both the original magnetic concentrate and in the natural gas used to fire the grate, no appreciable sulfur pickup was observed with the self-fluxed pellets. It has been reported, however, that the use of calcitic lime (CaO) can result in substantial sulfur pickup through formation of CaSO4 during pellet preheating. If sulfur is present, replacement of CaO by dolomitic lime should facilitate pellet desulfurization during, firing and cooling since MgSO4 is less stable and decomposes at a lower temperature (895° C in N2) than CaSO4 (1,149° C in N2).
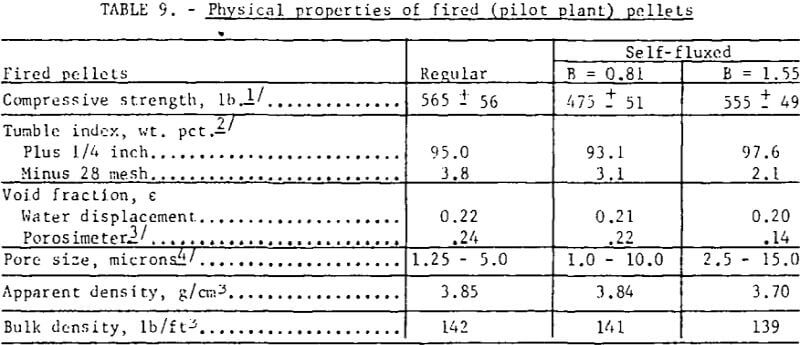
Pertinent data on the physical properties of the regular and the self-fluxed (pilot plant) pellets are listed in table 9. The compressive strengths were found to be comparable for both pellet types; the self-fluxed pellets, however, showed less variation from the average. Also, the self-fluxed pellets were somewhat more abrasion-resistant. Minor decreases were noted in the (pilot plant) pellet porosities with increasing lime additions; porosimeter measurements indicated that the self-fluxed, pellets have larger pore diameters but with a smaller percentage of surface-connected pores . As expected, the apparent and bulk densities were lower for self-fluxed pellets.
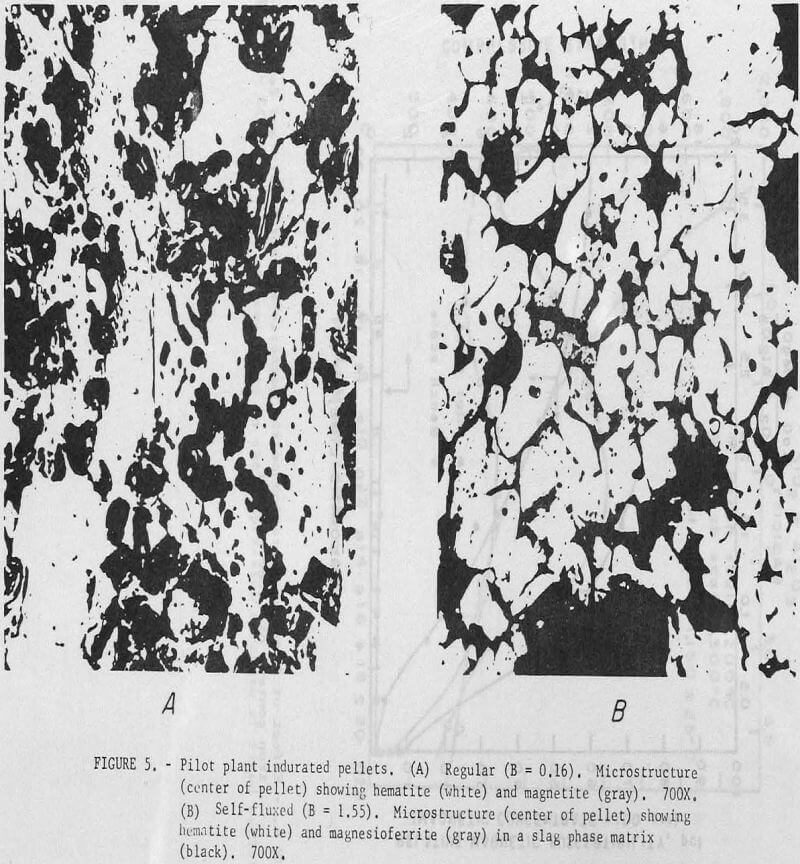
Photomicrographs of regular and self-fluxed (B = 1.55) pellets (fig. 5) show significant differences in microstructure. In regular pellets hematite particles retain their original shape; some partially oxidized magnetite grains may also be observed. Photomicrographs of seIf-fluxed pellets show subrounded to rounded hematite and magnesioferrite (or some solid solution of composition (FeO,MgO)·Fe2O3) grains cemented together by what appears to be a low melting temperature slag phase. It has not yet been determined whether the calcium present in these pellets is in solid solution with the magnesioferrite or associated with the glass phase.
Previous pilot plant induration tests under similar operating conditions with calcitic lime (CaO) resulted in fusion and loss of bed permeability at the higher pellet basicities (B = 1.6). The increased resistance of the dolomitic lime pellets to softening during induration can be attributed to the higher melting temperature of magnesioferrite (as compared to calcium ferrite). The pellet softening temperature would be an important consideration in both pelletization and blast furnace operation.
Metallurgical Properties of Fired Pellets
High-Temperature Linder Degradation
A major factor affecting the acceptability of self-fluxed pellets as a blast furnace raw material is their questionable behavior under reducing conditions. This behavior during the descent of the pellets through the upper stack of the blast furnace is generally evaluated by means of the standard Linder test which denotes the extent of pellet degradation. The tests also give a measure of the degree of deoxidation but this is considered of secondary importance.
Standard high-temperature (1,000° C) Linder tests on the fired (bench scale) pellets showed no discernible increase in disintegration with increased basicity (table 10). The exceptionally high Linder values can be attributed to the high strength and uniformity of the pellets. Deoxidation was significantly lower in the basicity range of 1.0 to 2.0 and was attributed to the lower effective porosity of these pellets.
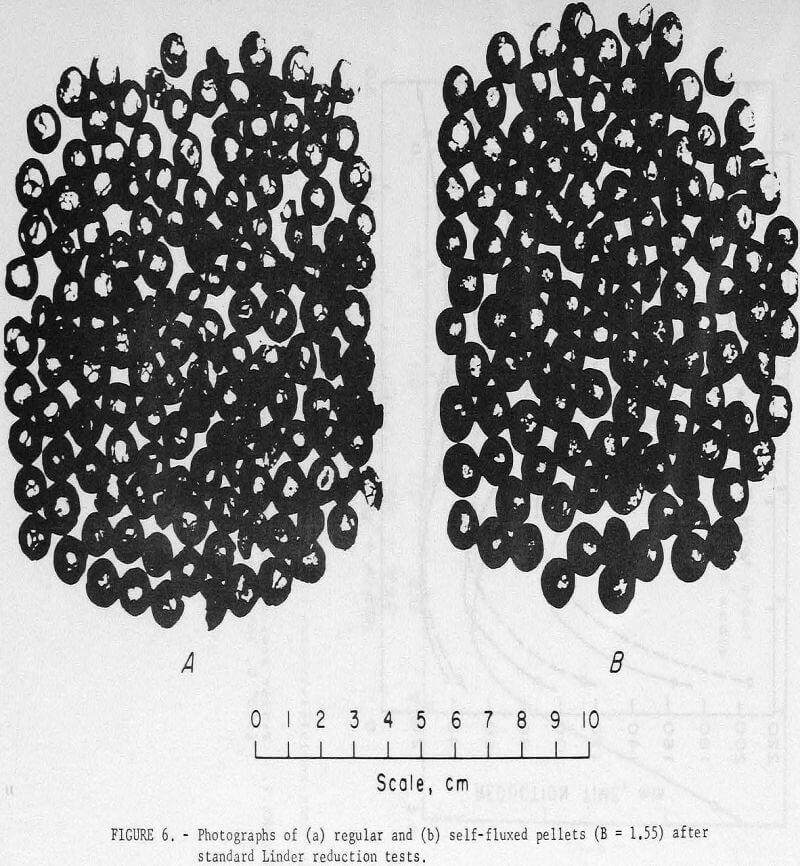
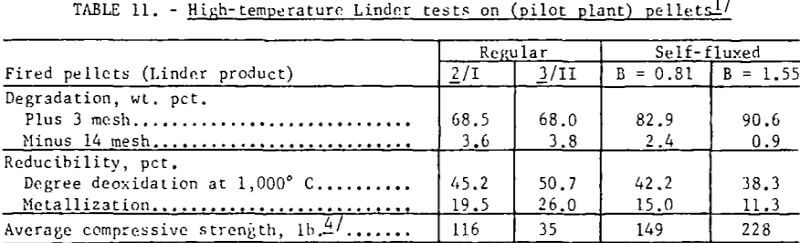
Self-fluxed pellets produced in the pilot plant, however, demonstrated considerably greater resistance to high-temperature reduction disintegration than the regular (pilot plant) pellets (table 11). As shown, Linder values obtained for the regular pellets were similar to those obtained for the commercial pellets. Photographs of regular and self-fluxed (pilot plant) pellets (B = 1.55) after Linder reduction (fig. 6) show less cracking in the self-fluxed pellets. The primary iron
minerals in the Linder products were α-iron and vustile; fayalite along with minor amounts of quartz, were present in the regular pellets but were not observed in the self-fluxed pellets. Microscopic examination showed that the fayalite was not well crystallized and appreciably more of this glass phase was present than indicated by the X-ray patterns.
Low-Temperature Linder Degradation
It has been recently reported that the standard high-temperature Linder test alone is not sufficient to evaluate the reduction disintegration resistance of self-fluxed pellets. Basic pellets that gave favorable results under high-temperature reducing conditions often showed extensive disintegration when reduced at low temperatures (600° C) under low reducing potential. Pellet degradation at such low temperatures was believed to take place during the early stages of magnetite formation. It has been postulated that the slag-binding phase present in self-fluxed pellets is weakened by reduction and cannot absorb the stresses caused, by the volume increase in the iron oxide during formation of porous magnetite. The bonding phases of most regular pellets are apparently not affected by the reduction in the early stages.
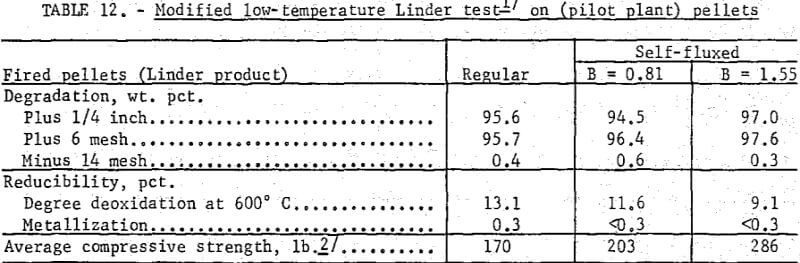
The newly created low-temperature Linder test was designed only for strength determination. As shown (table 12) neither the regular nor the self-fluxed pellets produced in this pilot plant study showed any appreciable breakdown under these low-temperature reducing conditions.
Modified Linder Degradation
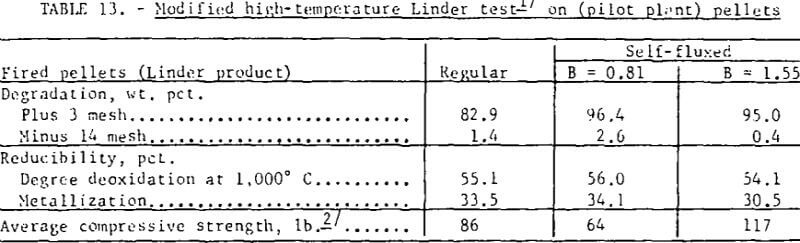
Reduction disintegration tests were also run on pilot plant regular and self- fluxed pellets with the Linder apparatus using a constant gas composition of 40 percent CO + 60 percent N2 throughout the test. With the exception of the change in gas composition, standard Linder test conditions were maintained. As shown (table 13) even with a higher reducing potential (higher reduction levels) the self-fluxed pellets had a greater resistance to reduction disintegration than the regular pellets.
Reducibility and Swelling Properties
The relative reduction rate of regular and self-fluxed pellets were measured under isothermal conditions using a weight-loss apparatus. Measurements were made on randomly selected pellets at a temperature of 900° C using a gas composition of 30 percent CO and 70 percent N2 and a velocity of 3 cm/sec. Preheating and cooling of the pellets were done under nitrogen. The degree of pellet swelling was also determined by measuring the pellet volume before and after reduction. Studies have shown that maximum swelling for regular North American pellets normally occurs towards the end of the wustite stage (30 to 40 percent reduction). Reduction above this level generally results in a gradual decrease in pellet size. In addition to normal swelling, some pellets, particularly those containing small amounts of CaO, have also been known to exhibit catastrophic swelling during the reduction of wustite to metallic iron with maximum volume expansion occurring at about the 70 percent reduction level. Bleifuss has shown that abnormal swelling in certain pellets is characterized by the growth of fine iron whiskers promoted by calcium in the magnetite lattice. He proposes that the limited mutual solubility of CaO and FeO produces an unstable condition and inhibits uniform nucleation of metallic iron. Pellets which exhibit such a high degree of swelling do not show normal topochemical reduction patterns.
None of the pellets used in this study exhibited any abnormal swelling behavior. Volume expansion measurements made at the 30 and 70 percent reduction levels were all less than 15 percent, while swelling of less than 20 percent is usually considered acceptable for blast furnace feed.
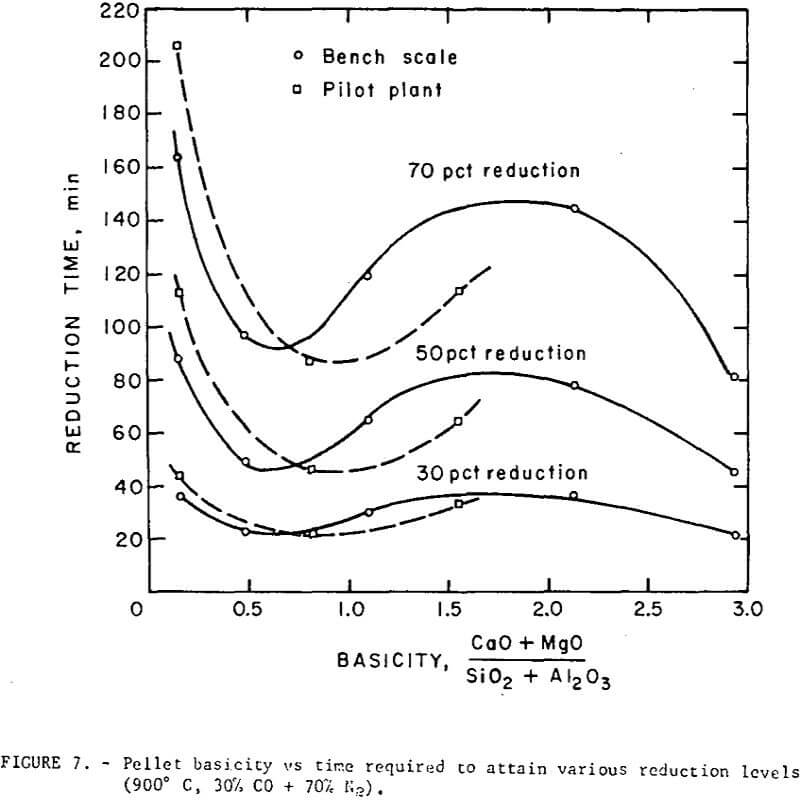
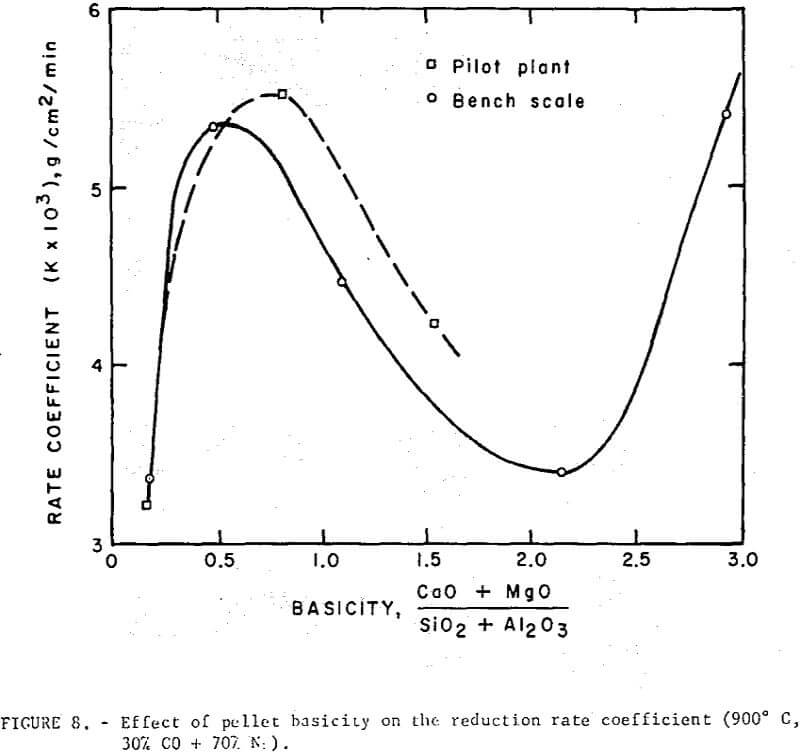
Reduction data on regular and self-fluxed pellets are summarized in figures 7 and 8. The reducibility index was expressed (a) as the time required to attain a specific reduction level (30, 50 and 70 percent reduction), and (b) in terms of a constant rate coefficient, k, defined as the grams of oxygen loss per square cm of surface per minute.

where: k = rate coefficient, g/cm²/min
ro = pellet radius, cm
do = pellet density, gm/cm³
%Fe = percentage of total iron in pellet
R = fractional reduction
t = reduction, time, min
The reduction data show no simple correlation between reducibility and the degree of basicity in the fired pellets. However, the level of lime concentration can greatly affect pellet reducibility through an increase or decrease in pellet porosity. Although it is difficult to separate the contributing effects of basicity and porosity, the self-fluxed pellets reduced under these conditions generally had a somewhat higher reducibility than the regular pellets. Maximum reduction rates occurred at a pellet basicity of about 0.5 and a minimum rate in basicity range of 1.5 to 2.5.
Magnesioferrite Reduction
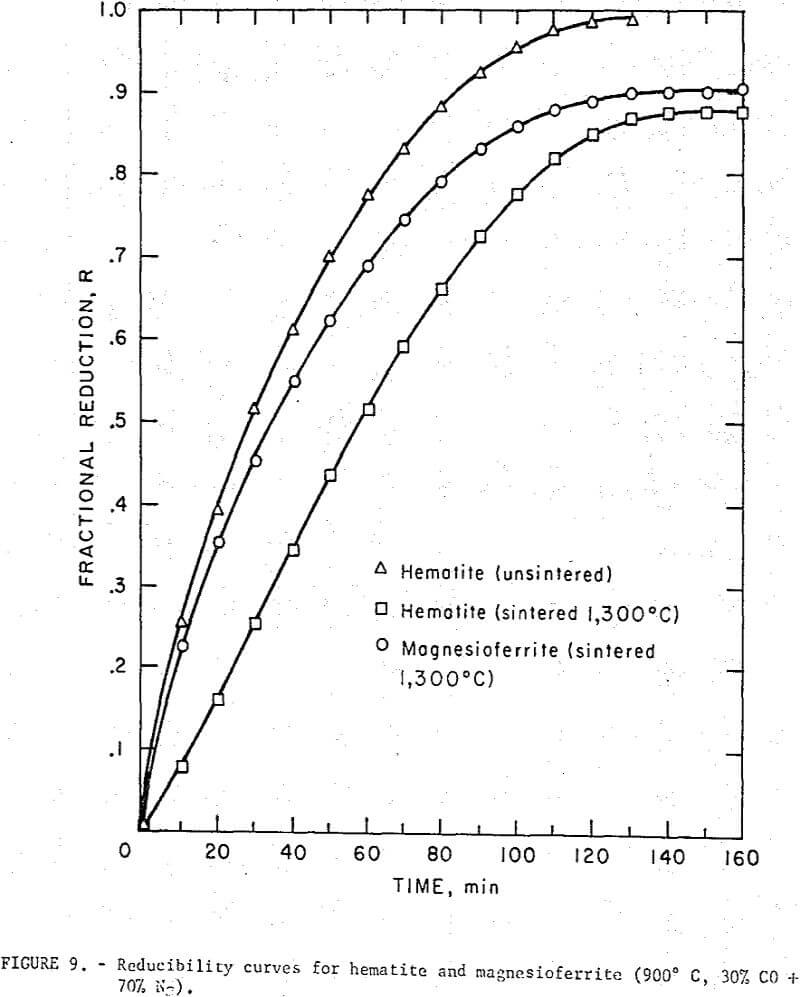
The presence of a magnesium and/or calcium ferrite phase in self-fluxed pellets will undoubtedly affect the rate of pellet reduction. Published reducibility studies on various calcium ferrite phases indicate that these ferrites generally reduce as readily as hematite and magnetite. Information on the reducibility of magnesioferrite, however, is not readily available. For this purpose reduction tests were run on magnesioferrite extrusions (3/8 inch diameter by 3/8 inch length) made from reagent grade Fe2O3 and MgO mixtures (1:1 mole ratio) and fired at 1,300° C. Verification of magnesioferrite was made by both X-ray diffraction and saturation magnetic moment measurements. Positive identification of magnesioferrite by X-ray diffraction was difficult because the d values were nearly identical to magnetite (Fe3O4) and maghemite (γ-Fe2O3). However, the absence of ferrous iron in the prepared magnesioferrite sample precluded the presence of magnetite and a number of other d values characteristic only of maghemite were missing, confirming that maghemite was also absent. Saturation magnetic moment (Ts) of the sample was measured at an applied field of 7,000 oersted at room temperature in a vibrating sample magnetometer. A measured Ts value of 28.5 emu/g was obtained which was close to the value of 27 emu/g reported for magnesioferrite. Published Ts values for maghenite and magnetite are 73.5 emu/g and 90 emu/g, respectively.
Reducibility curves for magnesioferritc and hematite extrusions are given in figure 9. The samples (~ 3 g) were sintered (1,300° C) under identical conditions and although small porosity variations may have existed the data showed that the reduction rates were comparable. The difference in the reducibility of sintered and unsintered hematite pointed out the influence of sample porosity.
Conclusions
Dolomitic ‘burnt’ lime was found to be a suitable additive for the preparation of self-fluxed iron oxide pellets. The mechanical and metallurgical properties of the dolomitic lime pellets (B = 1.55) were generally superior to the regular pellets. Their high resistance to softening during induration, attributable to the high melting temperature of magnesioferrite, would be an important consideration for selection of dolomitic lime for production of high basicity pellets from iron oxide concentrates containing larger amounts of silica. The question, however, of whether the cost of transporting this flux material to the plant site would offset the possible benefits received from reduction and smelting of these pellets in the blast furnace still remains to be answered.