Table of Contents
Having shown the difficulty of assaying so-called cyanide bullion and the extreme variations often found in the results, an investigation was undertaken to discover, if possible, the causes of these variations by a series of tests upon the specific action of various metals during cupellation.
What is the Effect of Zinc o Gold Assay
In order to test the effect of zinc, 48 assays were made in sets of 6, 2 rows of 3. The charge for the middle cupels consisted of 500+ mg. Au, 1,125 mg. Ag, 10 mg. Cu. To the end cupels 50, 75, 100 and 125 mg. Zn were added, and a set of each proportion was cupelled with 4 and 8 grams Pb. The cupels were assayed and showed the following gold contents in milligrams, the figures being arranged as the cupels stood in the muffle:
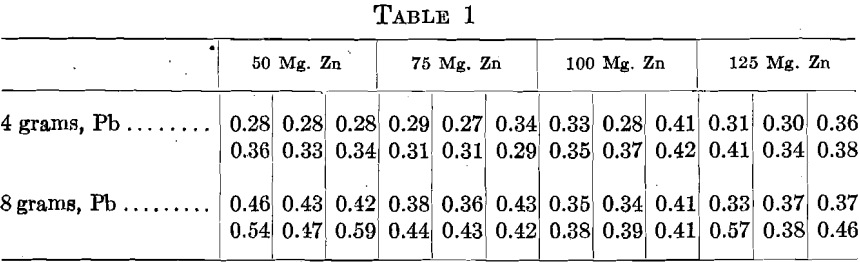
In many instances the zinc does not appear to affect the cupel gold, but various exceptions are apparent, generally in the cupels in the back row. With 100 and 125 mg. Zn, in some cases the Zn burned with its characteristic flame before it could alloy with the other metals. Also, less fusible oxidized lumps were observed during the cupellation and some cupels showed a scum.
In order to lessen the premature burning out of the Zn, an alloy of 2 parts Ag to 1 part Zn was used. A uniform charge of 500+ mg. Au, 300 mg. alloy (containing 100 mg. Zn), 925 mg. Ag and 20 mg. Cu. was used in sets of 9, 3 rows of 3. One set each was run with 4, 5, 8 and 10 grams Pb. In the table, the first column gives the relation of the cornet weight to the amount of Au taken and the second the amount of Au found by assaying the cupel, including beads, as well as Au actually absorbed by the cupel.
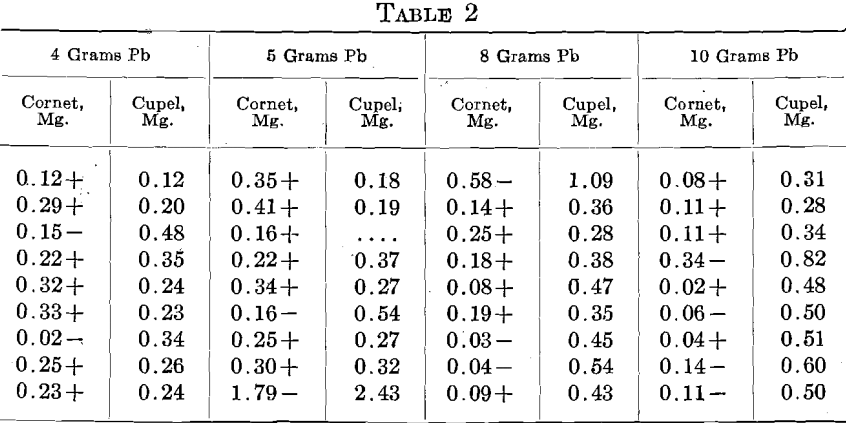
In a second set, 500 mg. of the alloy, containing 166 mg. Zn, and 790 Ag, were used, giving the following:
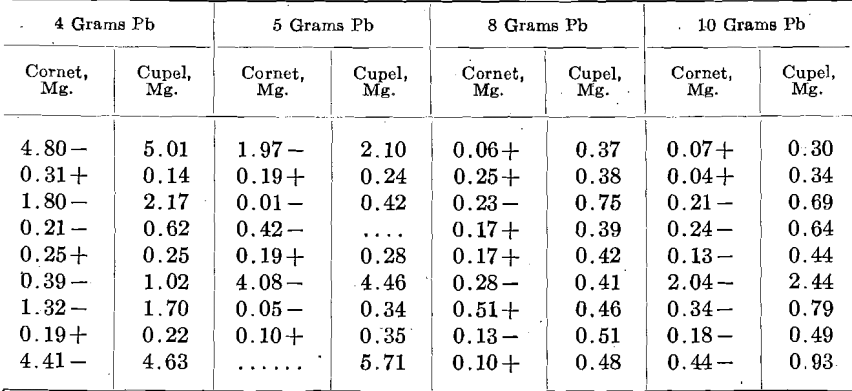
In these tests the pyrometer registered about 950° C. The figures are arranged as the cupels stood in the furnace.
From these figures, it is seen that Zn may be a most disturbing element. In various cases more or less infusible oxidized lumps were observed floating on the bead and these lumps entangled metal. In all probability this was due to the extreme preferential solubility of Au and Ag in Zn, the immiscibility of the resulting alloy with Pb, and its lower specific gravity; from all of which the Zn-Au-Ag alloy was formed, came to the surface of the bead and the Zn was oxidized without sufficient Pb to liquefy the ZnO. Eventually some of these lumps inclosing Au and Ag reached the cupel.
In a final comparison, the Ag-Zn alloy was used to introduce small amounts of Zn, three proportions—62.5, 85 and 125 mg. of alloy—being used. Each proportion was cupelled with 4, 5, 8 and 10 grams Pb, in sets of 9 cupels, 3 rows of 3, the middle cupel charge being without Zn as in the first test. This gives 36 rows, but 2 rows were lost. The 68 cupels with Zn yielded 21.39 mg. Au, and the 34 without, 10.70 or substantially half as much, thus showing that a small amount of alloyed Zn has no effect upon the cupel gold. However, the ZnO was not always properly absorbed by the cupel. With 125 mg. alloy there was a distinct tendency for the Zn-Au-Ag alloy to come to the surface. Curiously, this was also shown with the small amounts of alloy when 10 grams Pb were used. Evidently, the longer time required to oxidize such amounts of Pb offered sufficient opportunity for the Zn-Au-Ag alloy to form and gather at the surface. Therefore, the use of larger amounts of Pb to slag off Zn is not advisable.
Does Cadmium Impact Assaying of Gold
Having shown that Cd is often present in cyanide bullion, a few sets of 2 rows of 3 cupels were run, replacing the Ag-Zn alloy by Ag-Cd. One-half of the results obtained with each amount of Pb and Cd are given. The other results were similar.
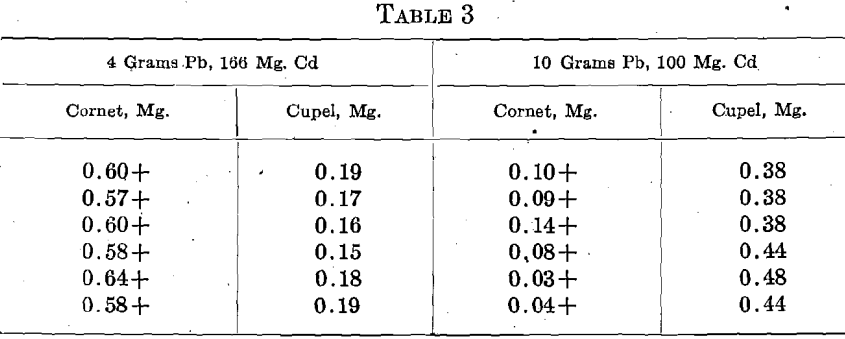
Aside from four beaded cupels, the results were uniform and very satisfactory, showing that Cd cannot be the cause of the wide variation so often found in cyanide bullion assays. No infusible lumps were observed in the cupelling beads, but in the 166 mg. Cd, 4 Pb sets, the cupels were heavily coated with the characteristic dark brown oxide of Cd.
The low Zn alloy runs were duplicated with the Cd alloy, and a most surprising result was obtained. Here also 2 rows were lost. The 68Cd cupels yielded 18.14 mg. Au, and the 34 without Cd yielded 9.28 mg. Au.
This is 0.21 mg. more than half of the yield of the Cd cupels and shows a distinct protective action by the Cd.
Further evidence of this protective action of Cd is furnished by a test in which an alloy of Cd-Zn-Cu-Ag was used. The charge in the end cupels was 500+ mg. Au, 500 mg. alloy, 740 mg. Ag. This gave approximately 40 mg. each of Cd and Zn and 25 mg. Cu. The middle cupels held 500+ mg. Au, 1,125 mg. Ag, 20 mg. Cu. These charges were run in sets of 9, 3 rows of 3, with 4, 5, 8 and 10 grams of Pb. In every row except one, the middle cupel yielded more gold than either end cupel. In the exception, the middle cupel yielded 0.06 mg. more than one end, and 0.02 less than the other, or 0.02 mg. more than the average of the ends. The 24 end cupels yielded 10.42 mg. Au, and the 12 middle ones 5.89, which is 0.68 mg. more than half of the ends. The following table, with 4 Pb, is characteristic, all the cornets being plus:

In some miscellaneous tests, 16 Cd end cupels yielded 3.58 mg. Au, and the 8 center ones, without Cd, 2.21 mg., 0.42 mg. more than half of the ends.
In many of the Cd calculations, the characteristic dark brown oxide was shown.
How does Copper Affect Gold Assays
An alloy of 8 parts Ag, 2 parts Cu, designated as 800 Ag, was used. The assay consisted of 450 +mg. Au, and 1,250 mg. Ag-Cu alloy. They were run with 5 and 10 grams Pb at two temperatures, stated as high and low, in 3 rows of 3 cupels.
The figures in Table 4 call for further and more elaborate investigation, but time was lacking to continue this work.
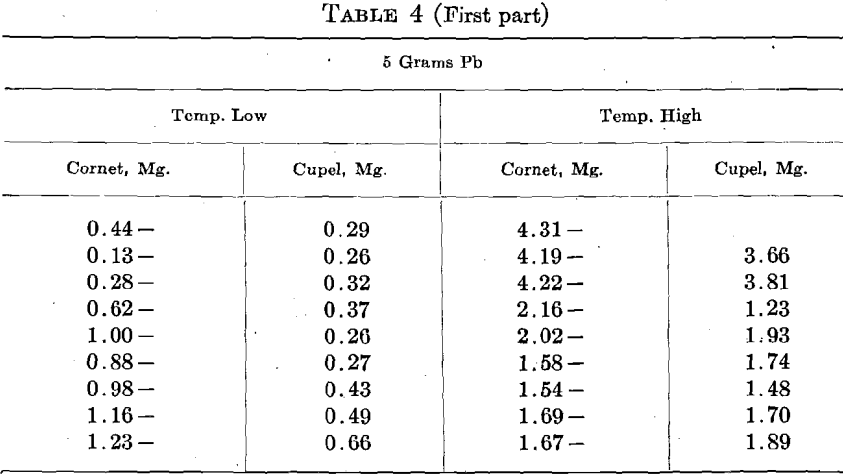
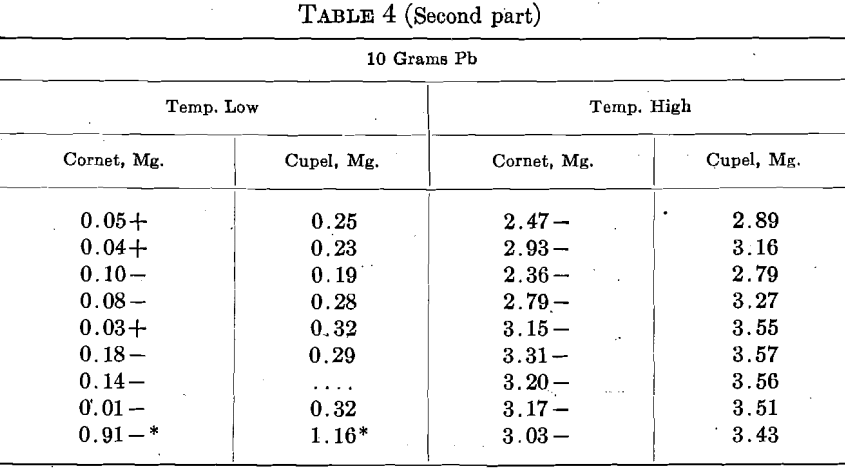
When Cu is present in bullion, we generally use more Pb and a higher temperature in cupellation. Both these conditions increase the cupel absorption of Au. In order to ascertain the effect of Cu per se upon the cupel absorption, 8 sets of 6 cupels, 2 rows of 3, were run with 5 to 7 mg. Cu, and 8 were run with 50 mg. Cu. Great care was exercised to have all of the other conditions as nearly as possible the same in every case, but a detailed inspection of the results indicates that the attempt was not altogether successful. The general temperature was shown by a pyrometer, and kept uniform, but the results show that the temperature of the beads did not conform to the pyrometer and varied considerably.
The cupel absorptions on the low Cu varied from 0.23 to 0.33 mg., and on the high Cu from 0.24 to 0.34 mg., but there was a very decided preponderance of low absorptions with low Cu and high ones with high Cu. The average absorptions were:

In order to keep the conditions the same, more Pb and a higher temperature were used on the low Cu, than were necessary, but the test clearly shows that Cu increases the cupel absorption.
The same fact is also shown in a general way by 26 rows with Cu in the ends, but without Cu in the centers. The Cu was alloyed with Ag and 2 proportions of Cu, 62.5 mg. and 125 mg. were each used with 4, 5, 8 and 10 grams Pb. The end cupels yielded 9.33 mg. Au, and the centers 4.12, or 0.545 mg., less than half of the ends.
What does Lead do to Gold Assaying Methods
Being the universal constituent of cupelling beads, the action of this metal is of the utmost importance. Many results have been published regarding its effects, but they are not, and could not really be expected to be, harmonious. There are so many variables involved that a tremendous amount of most painstaking work would be required to reach a quantitative expression of the relation of the weight of Pb in a bead to its effect upon the assay. I give here only a few illustrations, proposing to deal with the subject in detail in a separate paper on cupellation.
Under one set of conditions, 3 sets of 9 assays of fine gold showed the following cupel absorptions of Au, with differing amounts of Pb:
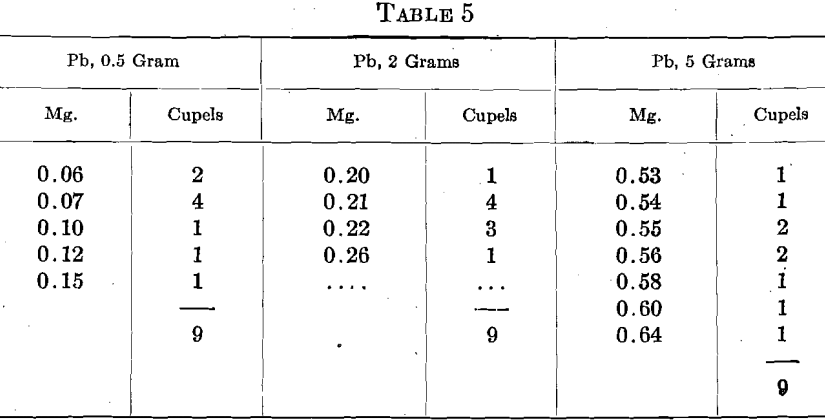
However, under a different set of conditions, and with 2 grams Pb, 8 sets of 6 assays of fine gold in one cupel gave 0.05 mg. absorption in one cupel, 0.06 mg. in 7 cupels, 0.07 mg. in 15 cupels, 0.08 mg. in 18 cupels, 0.09 mg. in 5 cupels and 0.11 in 2 cupels, total 48 cupels. Five sets of 6 assays in another cupel gave a preponderance of absorptions between 0.07 and 0.10 mg.
Even with 4 grams Pb, and on coin gold (900 Au, 100 Cu), the absorptions were less in another laboratory than they were on fine gold and 2 grams Pb in the first test.
Speaking in a very general way, under one set of conditions, doubling the Pb or increasing the pyrometer reading by 100° doubled the Au absorption, while both doubling the Pb and raising the pyrometer reading by 100° quadrupled the absorption of Au.
Effect of Silver og Gold Analysis
Intimately connected with the action of base metals is the protective action of Ag upon the absorption of Au by the cupel. Here, again, much work is required to reach quantitative results, but the general effect is shown by the cupel absorption of Au in the base metal assay and the corresponding Au assay.
Various samples were taken from a mass melt weighing 774.3 oz., made from 33 bars. On these 16 Au and 16 base assays were made. The absorptions in the gold assays varied between 0.17 and 0.27 mg., with the average 0.21 mg. The absorptions in the base assays varied between 0.64 and 1.00 mg. with the average 0.81 mg.
As a direct test of this question, sets of 6 cupels, 2 rows of 3, were run with varying proportions of Ag, and at 3 pyrometer readings. The total amount of gold adsorbed in each set, and the average per cupel, were, in milligrams:
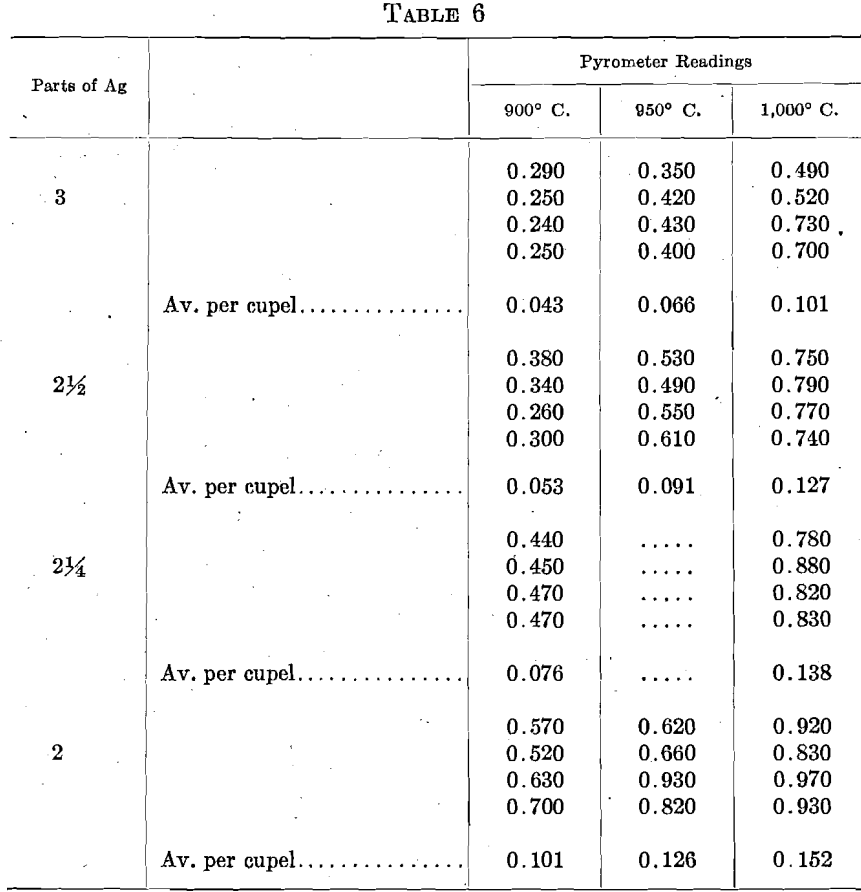
Under practical every-day conditions, the protection afforded by the Ag within ordinary parting conditions may be easily overbalanced by differences in other conditions.
I am greatly indebted to a former assistant, Mr. Scott Carter, for much painstaking effort in working out the data of this investigation.
Conclusions
In small amounts, Zn alone has no effect upon the cupel Au. In large amounts, the Zn-Au-Ag alloy is formed, comes to the surface and carries Au to the cupel. A tendency to do this occurs when small amounts of Zn are cupelled with large amounts of Pb.
In all the comparative tests Cd showed a distinct protection of the Au, even when Zn was also present.
The presence of Cu per se directly increases the absorption of gold, but its effect is intensified by the higher temperature and larger amount of Pb required.
The effect of Pb varies greatly with the surrounding conditions.
The presence of Ag is a good protection to the Au, but may be overbalanced by other conditions.
