The methods of assaying are best classed under two heads, Gravimetric and Volumetric, in the former of which the final results are weighed, whilst in the latter they are measured. A commoner and older division is expressed in the terms much used in practice —wet assays and dry assays. Wet assays include all those in which solvents, &c. (liquid at the ordinary temperature), are mainly used; and dry assays, those in which solid re-agents are almost exclusively employed. Dry assays form a branch of gravimetric work, and we shall include under this head all those assays requiring the help of a wind furnace. Wet assays, as generally understood, would include not only those which we class as wet gravimetric assays, but also all the volumetric processes.
Gravimetric Methods aim at the separation of the substance from the other matters present in the ore, so that it may be weighed; and, therefore, they must yield the whole of the substance in a pure state. It is not necessary that a metal should be weighed as metal; it may be weighed in the form of a compound of definite and well known composition. For example, one part by weight of silver chloride contains (and, if pure, always contains) 0.7527 part of silver; and a quantity of this metal can be as exactly determined by weighing it as chloride as by weighing it in the metallic state. But in either case the metal or its chloride must be pure.
Exact purity and complete separation are not easily obtained ; and methods are used which are defective in one or both of these respects. It is well to note that an impure product increases the result, whilst a loss of the substance decreases it; so that if both defects exist in a process they tend to neutralise each other. Of dry methods generally, it may be said that they neither give the whole of the substance nor give it pure; so that they are only calculated to show the amount of metal that can be extracted on a manufacturing scale, and not the actual quantity of it present.
Their determinations are generally rough and always low. The gold and silver determinations, however, will compare very favourably with any of the other processes for the estimation of these metals in their ores.
The calculation of the results of a gravimetric assay has already been referred to. If the result is to be stated as percentage, it may always be done by the following rule :—Multiply the weight of the substance got by the percentage of metal it contains, and divide by the weight of ore taken.
Gravimetric methods are divided into three groups: (i) mecchanical separations ; (2) dry methods; and (3) wet methods.
Mechanical Separations.—Under this head are classed the method of assaying tin ores, known as vanning, and the amalgamation assay for gold. A set of sieves to determine the relative proportion of powders of different degrees of fineness is sometimes useful. A set with 10, 20, 40 and 80 meshes to the inch is convenient.
Dry Assays.—An important distinction between wet and dry methods of assaying is, that in the former the substance is got into the liquid state by solution, whilst in the latter fusion is taken advantage of.
The difference between solution and fusion is easily illustrated : a lump of sugar heated over a candle-flame melts or fuses; suspended in water it dissolves. Many substances which are insoluble or infusible of themselves, become soluble or fusible when mixed with certain others; thus, in this way, solution is got with the aid of reagents, and fusion with the help of fluxes. For example, lead is insoluble in water, but if nitric acid be added, the metal rapidly disappears. It is convenient, but somewhat inaccurate, to say that the acid dissolves the lead. If the lead be acted on by nitric acid alone, without water, it is converted into a white powder, which does not dissolve until water is added; in this case it is obvious that the water is the solvent. The function of the acid is to convert the lead into a soluble compound.
Fluxes may act as true solvents. Fused carbonate of soda dissolves baric carbonate, and perhaps in many slags true solution occurs; but in the great majority of cases a flux is a solid reagent added for the purpose of forming a fusible compound with the earthy or stony minerals of the ore. Few of the minerals which occur in the gangue of an ore are fusible; and still fewer are sufficiently fusible for the purposes of the assayer, consequently the subject is one of importance, and it ought to be treated on chemical principles. An idea of the composition of some of the more frequently occurring rocks may be gathered from the following table, which represents rough averages :—
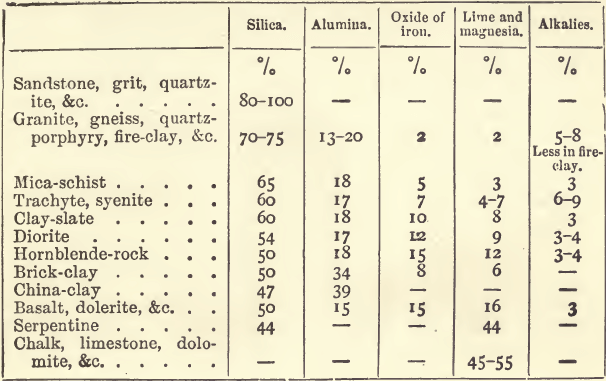
Silica itself, and the silicates of alumina, of lime, and of magnesia, are practically infusible; the silicates of soda, of potash, and of iron are easily fusible if the base (soda, potash, or oxide of iron) be present in sufficient quantity, and if, in the case of the iron, it is present mainly as lower oxide (ferrous silicate). The addition of lime, oxide of iron, or alkali to silicate of alumina results in the formation of a double silicate of alumina and lime, or of alumina and iron, &c., all of which are easily fusible. Similarly, if to a silicate of lime we add oxide of iron, or soda, or even alumina, a fusible double silicate will be formed. Thus lime, soda, oxide of iron, and clay, are fluxes when properly used ; but since lime, clay (and oxide of iron if there be any tendency to form peroxide), are of themselves infusible, any excess of these fluxes would tend to stiffen and render pasty the resulting slag. So, too, soda, which is a very strong base, may act prejudicially if it be in sufficient excess to set free notable quantities of lime and magnesia, which but for that excess would exist in combination as complex fusible silicates. There are many minerals which with but little soda form a glass, but with more yield a lumpy scoriacious mass. There are many minerals, too, which are already basic (for example, calcite), and which, when present, demand either a less basic or an acid flux according to the proportions in which they exist. For purposes of this kind borax, or glass, or clay with more or less soda may be used, and of these borax is by far the most generally useful. An objection to too basic a slag (and a very important one) is the speed with which it corrodes ordinary crucibles. These crucibles, consisting of quartz and clay, are rapidly attacked by lime, soda and bases generally.
In considering what is and what is not a good slag, certain chemical properties are of importance. If a mixture of many substances be fused and allowed to solidify in a crucible, there will be found some or all of the following. At the bottom of the crucible (fig. 4) a button of metal, resting on this a speise; then a regulus, next a slag made up of silicates and borates and metallic oxides, and lastly, on the top another layer of slag, mainly made up of fusible chlorides and sulphates.
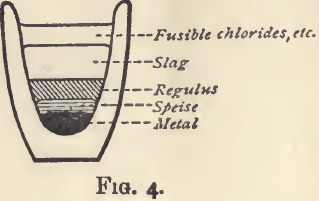
In assaying operations the object is generally to concentrate the metal sought for in a button of metal, speise or regulus, and to leave the earthy and other impurities as far as possible in the slag; whether there be one or two layers of slag is a matter of indifference; but the chemical action of the lower layer upon the speise, or regulus, or metal, is of great importance.
A regulus is a compound of one or more of the metals with sulphur; it is usually brittle, often crystalline, and of a dull somewhat greasy lustre. It is essential that the slag, when solid, shall be so much more brittle than the regulus, that it shall be easy to crumble, and remove it without breaking the latter; and it must not be basic. The effect of fusing a regulus with a basic slag is well seen when sulphide of lead is fused with carbonate of soda; the result is a button of metal (more or less pure), and a slag containing sulphides of lead and sodium ; and again, if sulphide of lead be fused with an excess of oxide of lead, a button of lead will be got, and a slag which is simply oxide of lead (with whatever it may have taken up from the crucible), or if a sufficient excess has not been used, oxide of lead mixed with some sulphide. When (as is most frequently the case) the desire is to prevent the formation of regulus, these reactions may be taken advantage of, but otherwise the use of a flux having any such tendency must be avoided. A good slag (from which a regulus may be easily separated) may be obtained by fusing, say, 20 grams of ore with borax 15 grams, powdered glass 15 grams, fluor spar, 20 grams, and lime 20 grams; by quenching the slag in water as soon as it has solidified, it is rendered very brittle.
Sulphide of iron formed during an assay will remain diffused through the slag, instead of fusing into a button of regulus, if the slag contain sulphide of sodium. The same is true of other sulphides if not present in too great a quantity, and if the temperature is not too high.
Speises are compounds of a metal or metals with arsenic. They are chiefly of interest in the metallurgy of nickel, cobalt, and tin. They are formed by heating the metal or ore in covered crucibles with arsenic and, if necessary, a reducing agent. The product is fused with more arsenic under a slag, consisting mainly of borax. They are very fusible, brittle compounds. On exposure to the air at a red heat the arsenic and the metal simultaneously oxidize. When iron, cobalt, nickel, and copper are present in the same speise, they are eliminated in the order mentioned.
Slags from which metals are to be separated should not be too acid ; at least, in those cases in which the metal is to be reduced from a compound, as well as separated from earthy impurities. Where the object is simply to get a button of metal from a substance in which it is already in the metallic state, but mixed with dross (made up of metallic oxides, such as those of zinc or iron), from which it is desired to separate it, an acid flux like borax is best; or, if the metal is easily fusible, and there would be danger of loss of metal by oxidation or volatilising, it may be melted under a layer of resin or fat. Common salt is sometimes used with a similar object, and is often useful. Under certain conditions, however, it has a tendency to cause the formation of volatile chlorides with a consequent loss of metal.
In the great majority of cases, the fusion of the metal is accompanied by reduction from the state of oxide ; in these the slag should be basic. It is not easy to reduce the whole of a reducible oxide (say oxide of copper or of iron) from a slag in which it exists as a borate or silicate ; there should be at least enough soda present to liberate it. When the object is to separate one metal, say copper, without reducing an unnecessary amount of another (iron) at the same time, a slag with a good deal of borax is a distinct advantage. The slag then will probably not be free from copper, so that it will be necessary to powder and mix the slag with some soda and a reducing agent, and to again fuse the slag in order to separate this residual metal. In all those cases in which the slag retains an oxide of a heavy metal, this cleaning of the slag is advisable, and in the case of rich ores necessary. Slags containing sulphides are especially apt to retain the more easily reducible metals.
The following are the ordinary and most useful fluxes :—
Soda.—The powdered bicarbonate, sold by druggists as “ carbonate of soda,” is generally used. It gives off its water and excess of carbonic acid readily and without fusion. Where the melting down is performed rapidly, the escaping gas is apt to cause trouble by frothing, and so causing waste of the material. Ordinary carbonate of soda, when hydrated (soda crystals), melts easily, and gives off its water with ebullition. It is unfit for use in assaying, but when dried it can be used instead of the bicarbonate. One part of the dried carbonate is equivalent to rather more than one and a half parts of the bicarbonate. From two to four parts of the flux are amply sufficient to yield a fluid slag with one part of earthy matter. This statement is also true of the fluxes which follow.
Borax is a hydrated biborate of soda, containing nearly half its weight of water. When heated it swells up, loses its water, and fuses into a glass. The swelling up may become a source of loss in the assay by pushing some of the contents out of the crucible. To avoid this, fused or dried borax may be used, in which case a little more than half the amount of borax indicated will suffice. Borax will flux almost anything, but it is especially valuable in fluxing lime, &c., and metallic oxides; as also in those cases in which it is desired to keep certain of the latter in the slag and out of the button of metal.
Oxide of Lead, in the form of red lead or litharge, is a valuable flux ; it easily dissolves those metallic oxides which are either infusible or difficultly fusible of themselves, such as oxides of iron or copper. The resulting slag is strongly basic and very corrosive; no crucible will long withstand the attack of a fused mixture of oxides of lead and copper. With silicates, also, it forms very fusible double silicates; but in the absence of silicates and borates it has no action upon lime or magnesia. Whether the lead be added as litharge or as red lead, it will exist in the slag as monoxide (litharge); the excess of oxygen of the red lead is thus available for oxidising purposes. If this oxidising power is pre-judicial, it may be neutralised by mixing the red lead with 1 per cent, of charcoal.
Glass: broken beakers and flasks, cleaned, dried, and powdered will do. It should be free from lead.
Fluor: fluor-spar as free as possible from other minerals, powdered. It helps to flux phosphate of lime, &c., and infusible silicates.
Lime: should be fresh and powdered. It must not be slaked. Powdered white marble (carbonate of lime) will do; but nearly double the quantity must be taken. One part of lime produces the same effect as 1.8 parts of the carbonate of lime.
Tartar and “ black flux,” are reducing agents as well as fluxes. The “black flux* which may be obtained by heating tartar, is a mixture of carbonate of potash and charcoal.
Reducing Agents.—The distinction between reducing agents and fluxes (too often ignored) is an important one. Fluxes yield slags; reducing agents give buttons of regulus or of metal. The action of a reducing agent is the separation of the oxygen or sulphur from the metal with which it is combined. For example, the mineral anglesite (lead sulphate) is a compound of lead, sulphur, and oxygen; by carefully heating it with charcoal the oxygen is taken away by the charcoal, and a regulus of lead sulphide remains. If the regulus be then fused with metallic iron the sulphur is removed by the iron, and metallic lead is left. The charcoal and the iron are reducing agents. But in defining a reducing agent as one which removes oxygen, or sulphur, from a metallic compound so as to set the metal free, it must be remembered that sulphur itself will reduce metallic lead from fused litharge, and that oxygen will similarly set free the metal in fused lead sulphide. There is no impropriety in describing sulphur as a reducing agent; but it is absurd to call oxygen one. Some confusion will be avoided if these substances and those which are opposite to them in property be classed as oxidising and deoxidising, sulphurising, and de-sulphurising agents. Most oxidising agents also act as de-sulphurisers.
