Dithiophosphate collectors are commonly used in the flotation of sulphide minerals. Studies of the interaction of a typical dithiophosphate collector with galena surfaces have been previously reported from these laboratories. These fundamental studies were carried out by employing phosphorus labeled radioactive dithiophosphate. Radioactivity determinations then could be used to measure readily and accurately the small concentrations of dithiophosphate that, are present under conditions of flotation processes.
Experimental
Materials: The mineral system was a ground synthetic mixture of galena, sphalerite, and quartz. The galena was from Galena, Kans., and contained 75 pct lead (theoretical 85.6 pct Pb for PbS); the sphalerite, from Ottowa County, Okla., contained 63 pct zinc (theoretical 67.2 pct Zn for ZnS); and the quartz from Klondike, Mo., analyzed as 99.97 pct SiO2.
Sulphur labeled radioactive di-(sec-butyl) dithiophosphate was prepared from S supplied by the Oak Ridge National Laboratory on allocation from the U. S. Atomic Energy Commission. The synthesis consisted of precipitating barium sulphate, reducing the barium sulphate to barium sulphide, acidifying to form hydrogen sulphide, and oxidizing the hydrogen sulphide with iodine to elemental sulphur.

The labeled sulphur was thoroughly mixed with 124 mg of nonradioactive carrier sulphur (USP flowers of sulphur), 66 mg of amorphous red phosphorus and 198 mg of carrier phosphorus pentasulphide, reagent grade, purified by extraction with
carbon disulphide. The mixture was heated slowly under dry nitrogen to 270°C and kept at 270° to 300 °C for 4 hr. T
Flotations: The flotations were carried out with 200 g of mineral in a Fagergren flotation machine having a 250-g cell. The galena, sphalerite, and quartz were crushed separately to pass a 10 mesh sieve, using a gyratory crusher for the quartz and a porcelain mortar and pestle for the galena and sphalerite. Synthetic mineral mixtures were made up with quartz to contain about 4.4 pct lead as galena or about 4.7 pct zinc as sphalerite, or to contain these amounts of galena and sphalerite together.
The sorption on the minerals was measured by counting thick mineral samples directly. A glass dish 5 cm in diam and 1.7 cm in height was used for low levels of radioactivity, and a cupped planchet 0.5 cm in height and 1.7 cm in diam was used for high-levels. These were filled to a constant height with mineral. For samples of constant density, the count observed can be assumed proportional to the count per unit weight under some standard conditions at infinitesimal thickness.
Results
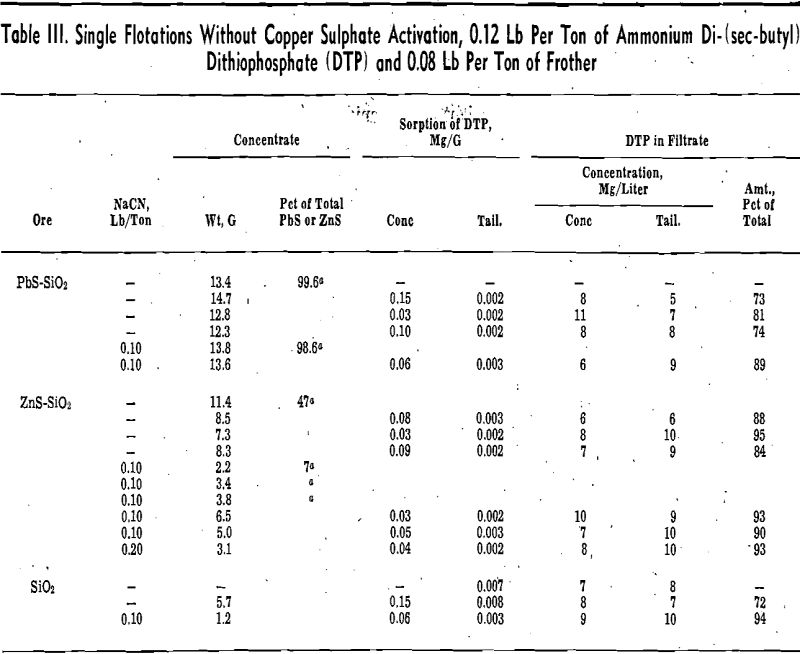
Flotation: The effectiveness of the separations accomplished was essentially as expected. The separation of galena from quartz under the conditions used was good (99.6 pct of the galena in the concentrate), while the separation of sphalerite from quartz was poor (47 pct of the sphalerite in the concentrate). Quartz was found in the concentrates from the galena-quartz and sphalerite-quartz flotations, and some quartz was also floated in the blank with quartz alone, but the amounts were not excessive in any of these experiments.
The addition of cyanide depressed the flotation of sphalerite from quartz, from 47 pct to 7 pct of the sphalerite in the concentrate, and the flotation of quartz in the blank, but the flotation of galena from quartz was not affected significantly. The weight of quartz floated along with the sphalerite and galena was not changed appreciably. The weight of quartz in the concentrate is calculated from the total weight of concentrate less the weight of galena or sphalerite based on a total of 11.5 g of galena or 14.9 g of sphalerite taken for flotation.
Sorption: A major part, 72 to 95 pct, of the di-thiophosphate present remained in solution in all of the experiments. The concentration in the solutions from the concentrate was in fair agreement with the concentration in the solutions from the tailing. The differences observed were not consistent.
The dithiophosphate sorption on the concentrate varied considerably between experiments. Values of 0.03, 0.10, and 0.15 mg per g were obtained in the flotation of galena without cyanide, and the values observed in the remaining experiments were all within the same limits. Except for the quartz flotation without cyanide, the dithiophosphate sorption on the tailing was relatively more consistent, 0.002 to 0.003 mg per g, and was lower than the sorption on the concentrate by a factor of at least ten.

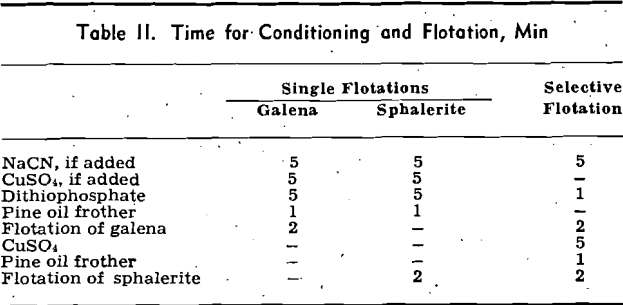
Selective Flotations: The data obtained in the selective flotations are shown in Table V. Each selective flotation experiment consists of two parts: part G, the flotation of galena in the presence of cyanide, and part S, the flotation of sphalerite from the tailing of part G using copper sulphate to activate the sphalerite.
Flotation: Reasonably good separation of galena was obtained by using cyanide and zinc sulphate to depress the sphalerite flotation. One flotation made without zinc sulphate had a considerable sphalerite contamination in the galena, the separation appearing to be of such poor quality that no assays were made.
It would have been of considerable interest to measure directly the sorption of dithiophosphate on quartz, galena, and sphalerite separately in the rougher concentrates. Attempts to separate the quartz from the galena or sphalerite were unsuccessful. Separation by flotation could not be used because dithiophosphate would desorb. A separation was achieved by gravity in acetylene tetrabromide, but it was found that dithiophosphate also desorbed in this medium. An attempted magnetic separation was likewise unsuccessful.
The selective flotations at high alkalinity using lime with the copper sulphate did not appear to involve the bulk precipitation of copper dithiophosphate. This is seen most obviously by comparing the solution concentrations of experiment 4S (pH 10.8) with experiment 2S (pH 6.8). In experiment 4S, a considerable portion of the dithiophosphate remained in solution. O
