Table of Contents
Destructive distillation was demonstrated to be a technically feasible method of disposing of and obtaining potentially valuable products from scrap automobile and truck tires.
The relative amounts of the various products were shown to be highly dependent on the temperature of carbonization. Highest yields of liquid products were obtained at 500° C and highest yields of solid and gaseous products were obtained at 900° C.
The compositions of the liquid and gaseous products were also dependent on the test temperature. The liquid components contained a higher percentage of aromatic components at 900° C than at 500° C. The gases from the high-temperature tests contained a higher percentage of hydrogen and hence had a lower heating value than those from the low-temperature tests.
Analyses and characterizations of the heavy oils by a standard procedure for coal tars, by gas chromatography, and by mass spectrometry indicate a mixture of a large number of components. In the volatile portion of the oils, 102 peaks were observed by gas chromatography.
The light oils from 500° C tests contained more highly saturated hydrocarbons than those from 900° C tests. The light oils from high-temperature tests contained substantial amounts of benzene and toluene.
Rubber vulcanizates containing tire cord fabric produced the same type of products that were produced from tests on rubber vulcanizates containing no fabric.
Residues from all tests were friable carbonaceous materials. Only a small amount of residue was obtained from the carbonization of a synthetic styrene-butadiene emulsion copolymer.
The volatile-matter content of the residue was related to the carbonization temperature. At 900° C, less volatile matter remained in the residue than at 500° C.
Carbonization temperature had little effect on the sulfur content of the residue. Nearly all of the sulfur remained in the residue regardless of test temperature.
The disposal of used tires of all types is an ever-increasing national problem. The shipments of replacement passenger, truck, and bus tires have been increasing at a rate of about 7 percent per year to a high of 215 million units in 1968. The rubber industry consumed 5.5 billion pounds of new rubber (natural and synthetic combined) and in excess of 400 million pounds of fabric in 1968.
Only a small proportion of these tires are retreaded. Many are burned haphazardly in open air dumps, a condition which cannot continue because air pollution laws are growing stricter every year. Figure 1 shows the pollution caused by a fire in a pile of used tires, and figure 2 shows a pile of used tires at the Firestone Tire and Rubber Company, Akron, Ohio, plant. Some of the scrap tires are buried, but they make poor land-fill because they do not readily degrade.

Cognizant of this disposal problem, The Firestone Tire and Rubber Company of Akron, Ohio, entered into a cooperative agreement with the Bureau of Mines to determine the feasibility of carbonization (destructive distillation) of used tires as a means of solving the pollution problem and improving the economics of disposal by recovering usable products. The Bureau of Mines Coal Carbonization group at Pittsburgh, Pa., has a unique pilot plant, known as the Bureau of Mines-American Gas Association (BM-AGA) tester, which is used to determine the carbonizing properties of coal. The pilot plant, used in this investigation, has a complete product recovery train and provides sufficient solid, gaseous, and liquid products for analysis and evaluation.

Acknowledgments
The authors acknowledge indebtedness to J. R. Laman, Manager of Environmental Engineering of Firestone Tire and Rubber Company, who was instrumental in initiating the program and the following personnel who assisted in conducting the carbonization tests and collection and analysis of the products: R. Gutierrez, chemical assistant at Firestone; and C. Ortuglio, research chemist, F. W. McCown, physical science technician, G. Serapiglia, first operator, research equipment, and W. L. Krobot, research equipment operator helper, of the Bureau of Mines carbonization staff.
Description and Analyses of Raw Materials
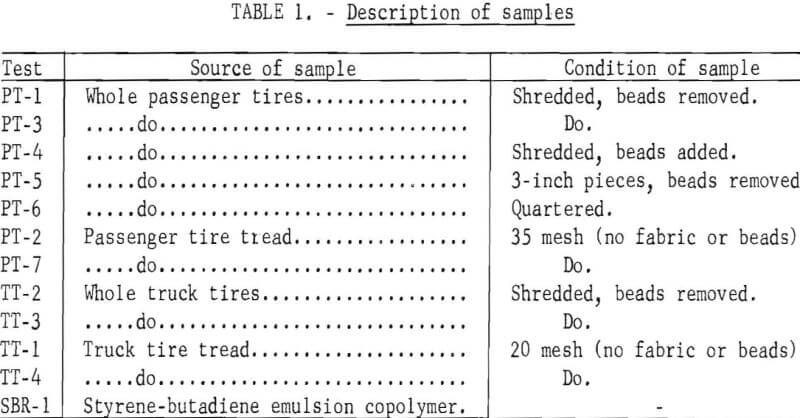
The scrap tires charged were prepared by The Firestone Tire and Rubber Company at its Akron plant. Description of the various types of raw material and the order of presenting data in subsequent tables are given in table 1. PT is used as a prefix to designate passenger tires, TT, truck tires, and SBR, the styrene-butadiene emulsion copolymer used in the manufacture of synthetic tires. The shredded whole passenger tires include the fabric with the bead removed mechanically. In one test (PT-4), 25 pounds of bead was added to the shredded passenger tire charge. In another test (PT-6), uncut whole passenger tires were obtained from a disposal pile at a neighborhood gasoline station and were cut into large pieces. The 35-mesh scrap passenger tires include only the rubber with the bead removed mechanically and the fabric removed by treatment with aqueous calcium chloride solution at elevated temperatures. The shredded truck tires include the fabric with the bead removed mechanically. The 20-mesh scrap truck tires contain only the tread rubber with the fabric and bead removed mechanically.
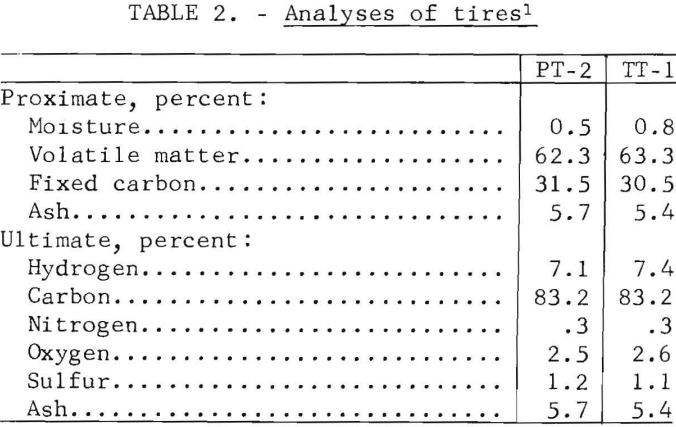
The proximate and ultimate analyses of the passenger and truck tires without fabric or beads are given in table 2.
Equipment and Procedure
Carbonizing properties were determined by the Bureau of Mines-American Gas Association (BM-AGA) method. The pilot plant is shown pictorially in figure 3 and schematically in figure 4, and consists essentially of an electric furnace, cylindrical steel retort, condensing and scrubbing train for recovery of products, and gas metering and sampling devices.
As shown in figure 4, the electric furnace (1) is 26 inches in internal diameter and 48 inches deep. A cast refractory block, 18 inches in diameter and 11-½ inches high, is located at the bottom-center of the furnace to support the retort. The furnace is heated electrically by nickel-chromium resistors, evenly spaced in the furnace wall. The retort (2) is constructed of 16-gage mild steel in the wall and 10-gage steel in the top and bottom. It is 26 inches high and 18 inches in diameter. The effluent gases and vapors pass from the retort through a 2-inch-diameter pipe, located at the top-center,

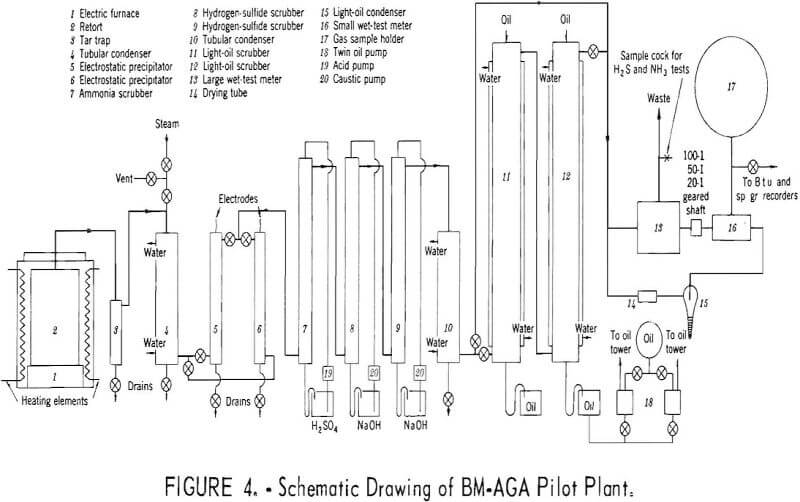
and enter an air-cooled trap, where the heavier oils are collected. The gas and vapors then pass through a water-cooled tubular condenser, where they are cooled to room temperature and additional heavy oil is collected. The last traces of the heavy oil mist are removed by one of the alternate electrostatic precipitators. The gas then passes successively through packed scrubbers, where ammonia is removed with sulfuric acid, and carbon dioxide and hydrogen sulfide with caustic soda. In these series of tests, the refrigerated water condenser and the oil scrubbers were bypassed and the gases passed directly to the large and small meters, which are geared together so that sampling of the gas is continuous and automatic. Dependent on the carbonizing temperature, the gear ratios are set so that 99 percent of the gas is flared and 1 percent passes through an ice-water cooled condenser and a condenser immersed in acetone and solid carbon dioxide to remove the light oil. The quantity of light oil recovered, which is from only that portion of the gas that passes through the small meter, is calculated to a total gas yield basis. The gases then pass through the small meter to the gasholder. Steam, which is shown connected atop condenser, is used to remove heavy oil from the air-cooled condenser, water-cooled condenser, and piping at the conclusion of the test.
In preparing for a test, the retort, with the bottom open, is inverted and filled with a weighed charge, and the bottom is welded into place. About 100 pounds of tires were used for each test, which gives a bulk density of about 30 pounds per cubic foot. A charge of this weight yields sufficient products for analysis and processing.
The retort is inserted into a carbonizing furnace, which is preheated 50° C above the desired temperature to compensate for heat loss during this operation. After an initial recovery period of about 15 minutes, the retort wall is held at a constant temperature (±5° C) by automatic control of the power input to the furnace. The control is activated by a thermocouple located on the retort wall midway between top and bottom.
The test is discontinued when the evolution of the gas reaches a rate that will no longer support combustion at the waste gas burner. Usually a test lasted 8 to 12 hours depending upon the test temperature.
Results and Discussion
Yields of Carbonization Products
In computing yields from BM-AGA carbonization test, U.S. gallons (231 cubic inches) and short tons (2,000 pounds) are used Heavy oil is the material collected in the following units of the BM-AGA recovery train: Air-cooled condenser, water-cooled condenser, and electrostatic precipitator. Light oil is the material collected from an aliquot portion of the gas in ice water and Dry Ice acetone condensers after the heavy oils are removed and the gas has passed through the acid and caustic scrubbers. The residue is the total material remaining in the BM-AGA retort after the test has been completed. The total gas is that obtained after passing through the BM-AGA recovery train and is corrected to 60° F and 30 inches of mercury pressure.
Carbonization temperature of 500° C was selected to obtain large liquid yields and 900° C for increased gas yields. To determine the effect of increasing temperature on increasing gas yields after liquid products were recovered, tests were started at temperatures as low as 140° C and increased to a final temperature as high as 926° C. Table 3 gives the yields of carbonization products on a percentage and per ton of tires basis.
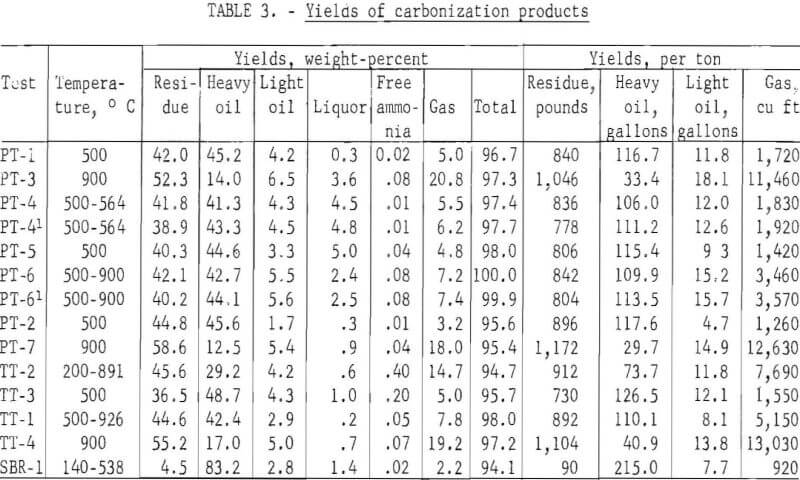
Three tests on passenger tires at 500° C yielded 806 to 896 pounds per ton of residue, 122.3 to 128.5 gallons of oil, and 1,260 to 1,720 cubic feet of gas. One of the samples was shredded without beads, another, 3-inch chunks without beads, and the third, 35-mesh scrap passenger tires without fabric or beads. It is apparent that the preparation of the samples and the composition, whether with or without fabric, had little effect on the yields of carbonization products. However, the yields of carbonization products were related to the carbonization temperature. At 900° C, the yields of residue were 1,046 and 1,172 pounds per ton; oil, 51.5 and 43.6 gallons; and gas, 11,460 and 12,630 cubic feet. In another test (PT-6), the temperature of carbonization was held at 500° C until most of the liquid products were removed and then increased rapidly to 900° C to increase the yield of gas. The yield of liquid products (129.2 gallons) was similar to the 500° C test, but the yield of gas was higher (3,570 cubic feet). Scrap truck tires without fabric or beads, shredded to 20-mesh size, carbonized at 500° C, yielded 730 pounds of residue, 138.6 gallons of oil, and 1,550 cubic feet of gas. The yield of residue was lower and oil higher for the truck tires than for the passenger tires, which may be attributed to the higher percentages of natural rubber in the truck tires. At 900° C, the truck tires yielded 1,104 pounds of residue, 54.7 gallons of oil, and 13,030 cubic feet of gas. In another test in which the initial temperature was 500° C and the final temperature 926° C, the residue yield was 892 pounds; oil, 118.2 gallons; and gas, 5,150 cubic feet per ton. Thus, it is apparent that by varying the carbonizing temperature the yields of carbonization products can be changed. One test was made on a styrene-butadiene emulsion copolymer at an initial temperature of 140° C and a final temperature of 538° C. A low residue yield of 90 pounds per ton, high oil yield of 222.7 gallons, and a low gas yield of 920 cubic feet were obtained.
Residue
Proximate and ultimate analyses of the residue, heating values and surface areas by the BET method are given in table 4.
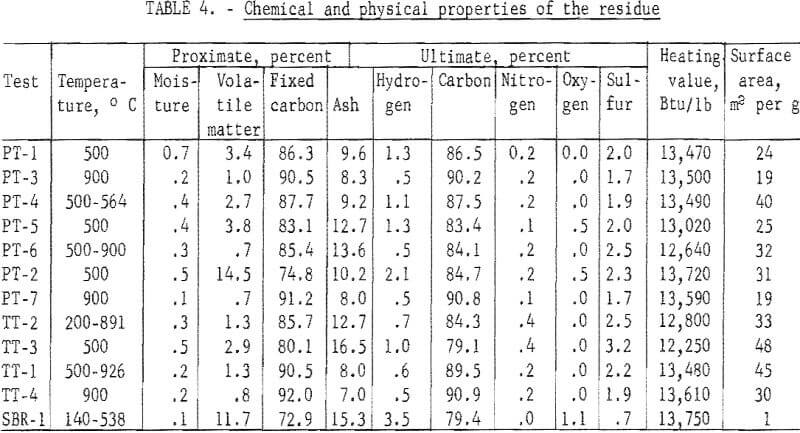
Except for one test (PT-2) at 500° C, in which the residue was not completely devolatilized, the volatile matter was related to the final carbonizing temperature. At 500° C, the volatile matter ranged from 2.9 to 3.8 percent, and at 900° C, from 0.7 to 1.0 percent. The tests with initial temperatures of 500° C and final temperature at about 900° C yielded residue with 0.7 and 1.3 percent volatile matter. Another test with an initial temperature of 200° C and a final temperature of 891° C yielded a residue with a volatile matter of 1.3 percent. The sulfur content of the residue was not related to the carbonization temperature and ranged from 1.7 to 3.2 percent. Similarly, the heating value of the residue was not related to carbonization temperature and ranged from 12,250 to 13,610 Btu per pound. The surface area of the residue from the passenger tires ranged from 19 to 40 square meters per gram, with the residue from the two 900° C tests both at a lower level. The surface area values for the truck tires were generally higher and ranged from 30 to 48 square meters per gram. The surface area of the residue from the SBR mixture was extremely low at 1 square meter per gram.
Gas
The determined values for the specific gravities and heating values of the gas and the calculated values corrected for the carbon dioxide and hydrogen sulfide removed by the caustic scrubber are given in table 5, and the chromatographic analysis in table 6. A two-column technique was used for the gas analyses. Hydrogen, oxygen, nitrogen, and methane were determined with a 64-inch-long, ¼-inch-diameter column, containing 5A molecular sieve, and was maintained at 30° C. Detection was with a thermal conductivity cell, and the carrier gas was helium flowing at a rate of 50 ml/minute. The remainder of the components were eluted on a 25-foot-long, ¼-inch-diameter column containing 20 percent 2,5-hexanedione on 30- to 60-mesh Kromat. The column was maintained at 0° C; helium at 50 ml/minute was the carrier gas, and a thermal conductivity cell was the detector. Sample size was 2 ml. Because of the unreliability of the experimentally determined hydrogen concentration due to the large percentage of hydrogen in the gas and a hydrogen leakage problem with the septum on the sample tubes, all gases were calculated to a hydrogen-free basis. The hydrogen concentration was then calculated by utilizing the experimentally determined heating value of a cubic foot of the make gas and the heating values of the hydrogen constituents of the make gas. Heating values of the component gases were taken at 30 inches of mercury pressure, 60° F, and saturated with water vapor.
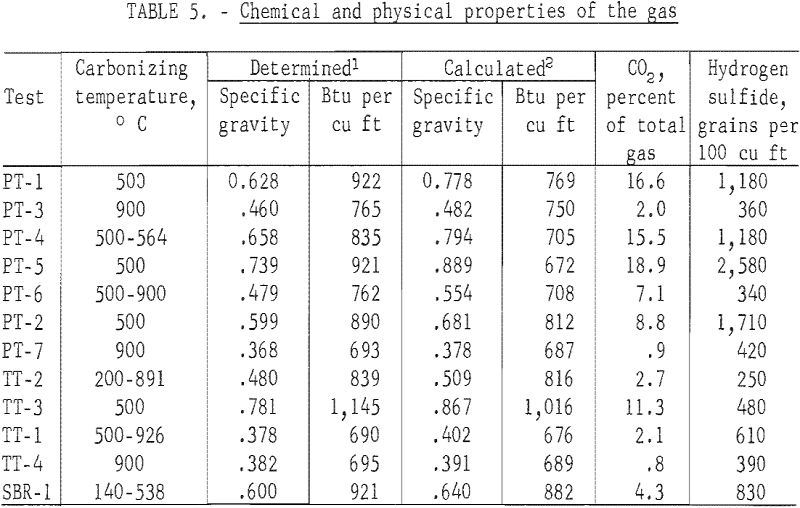
The heating value of the gases were related to the carbonizing temperature for the three passenger tire tests carbonized at 500° C; the determined heating value ranged from 890 to 922 Btu per cubic foot and for the two tests at 900° C, 765 and 693 Btu. For the truck tires, carbonized at 500° C, the gas had a determined heating value of 1,145 Btu per cubic foot and at 900° C, 695 Btu. The heating value of the gases for both the passenger and truck tires heated from an initial temperature of 500° C and a final temperature of about 900° C were similar to their respective 900° C values. Fourteen components were identified in the gases. The chemical composition of the gases were also related to carbonizing temperature. The higher calorific gases from the 500° C tests contained greater percentages of the higher calorific components from ethane through the pentenes and less hydrogen and methane than those from the 900° C tests.
Heavy Oils
Three methods were used for the analysis and characterization of the heavy oils. Standard procedure for the analysis of coal tars, gas chromatography, and mass spectrometry.
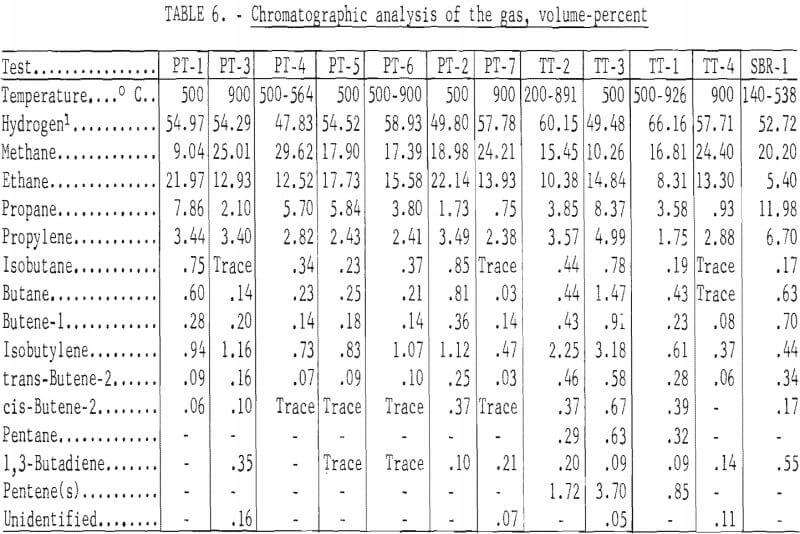
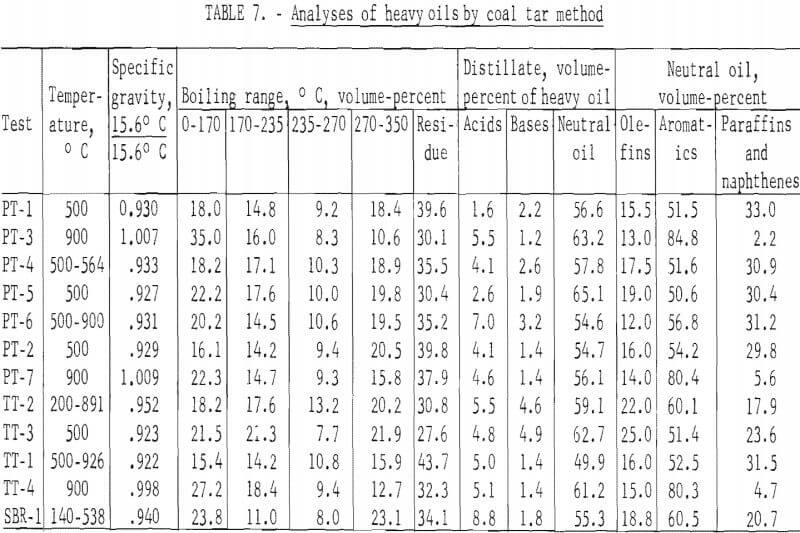
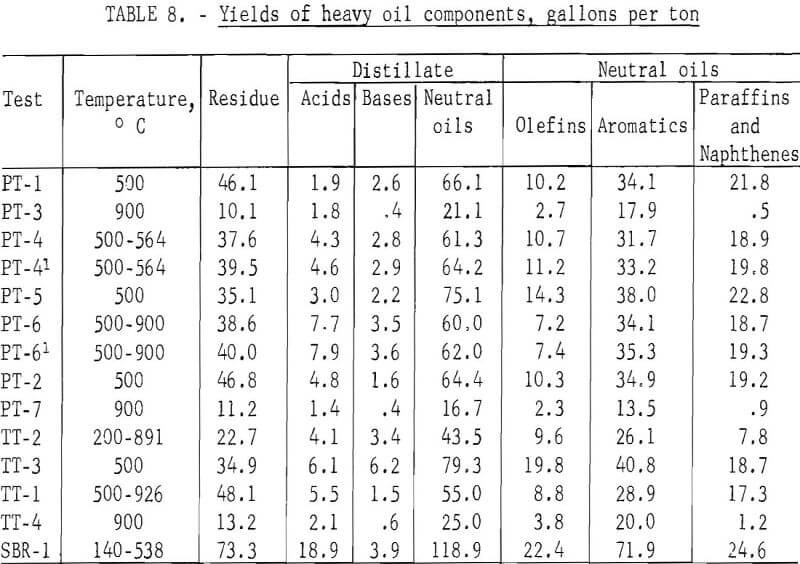
The standard procedure for the analysis of tars from coal carbonization is described in detail by Kester and coworkers and as specifically applied to BM-AGA carbonization by Walters and coworkers. Table 7 gives the distillation range of the heavy oils and the content of acids, bases, and neutral oils in the distillate and olefins, aromatics, and paraffins and naphthenes in the neutral oils. Table 8 gives the yields in gallons per ton of scrap rubber carbonized. By this method of analysis, the distillation range and content of acids, bases, and neutral oils in the distillate showed no significant trend. However, the effect of temperature of carbonization is apparent in the aromatic and paraffins and naphthene content of the neutral oils. For those tests which were run at 500° C and those started below this temperature to remove most of the oil before increasing the temperature to increase gas yields, the aromatic content ranged from 50.6 to 60.1 percent, and the paraffin and naphthene content from 11.9 to 33.0 percent; whereas, for the 900° C tests, the range of these components were 80.3 to 84.8 and 2.2 to 5.6 percent, respectively. Since the total yields of heavy oils were effected by the temperature of carbonization, the yields, per ton of scrap tires, of residue and all the various components in the distillate also show the effect of carbonization temperature. For the tests at 500° C and those tests in which most of oils were recovered before raising the temperature, the average yield in gallons per ton was as follows: Distillation residue, 39.2; acids, 4.7; bases, 3.0; neutral oils, 63.7; olefins, 11.4; aromatics, 33.9; and paraffins and naphthenes, 18.3. The average yields in gallons per ton for the 900° C tests were as follows: Distillation residue, 11.5; acids, 1.8; bases, 0.5; neutral oils, 20.9; olefins, 2.9; aromatics, 17.1; and paraffins and naphthenes, 0.9.
The following procedure was used for the gas chromatographic analysis of the heavy oils. A dual flame ionization chromatograph was used and the heavy oils were eluted on a 14-foot-long, ¼-inch-diameter column containing 20 percent tricresyl phosphate on 60- to 80-mesh Chromosorb P. The column was backflushed after the emergence of styrene onto a 4-foot-long, ¼-inch-diameter column, containing 5 percent SE-30 on Chromoport to obtain the components that had not been eluted on the tricresyl phosphate column. Other pertinent operation conditions were as follows:
Carrier and flow……………………………………………………helium at 50 ml/minute.
Temperature of injection port……………………………………………300° C.
Temperature at detector……………………………………………………350° C.
Column temperature programing…………………..4 minutes at 35° C, 2° C/minute to 125° C, then held at 125° C.
Sample size………………………………………………………………………..5µl
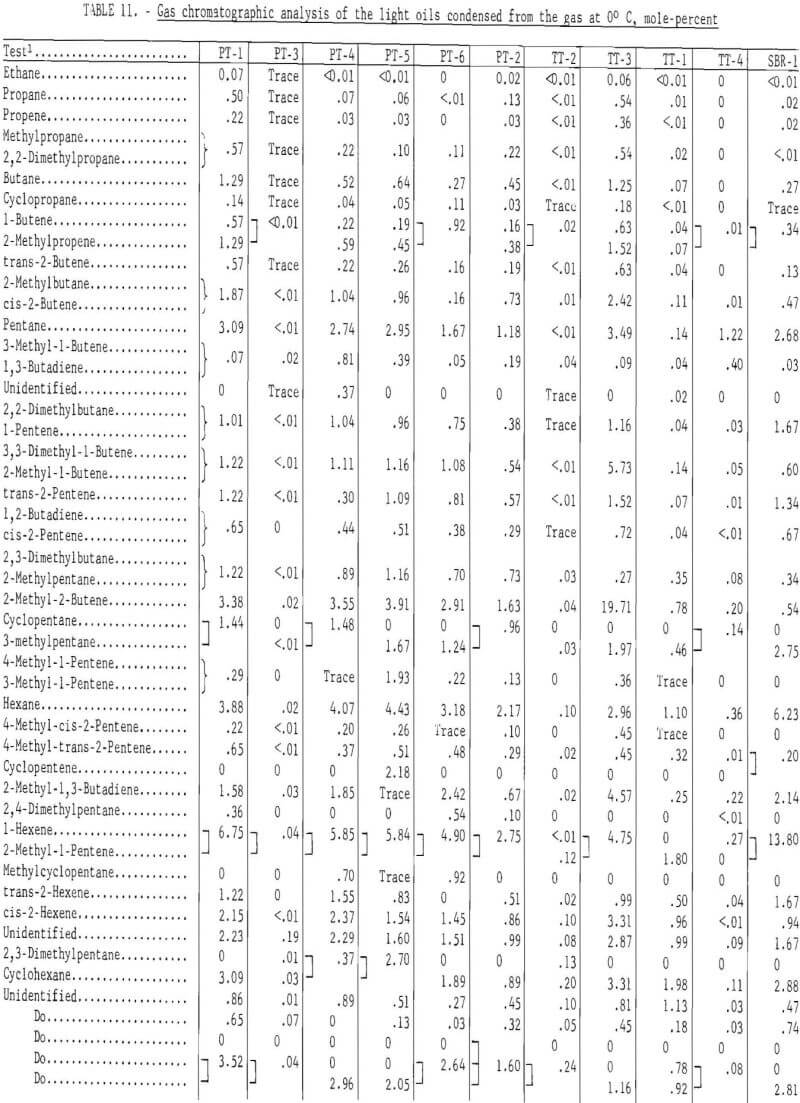
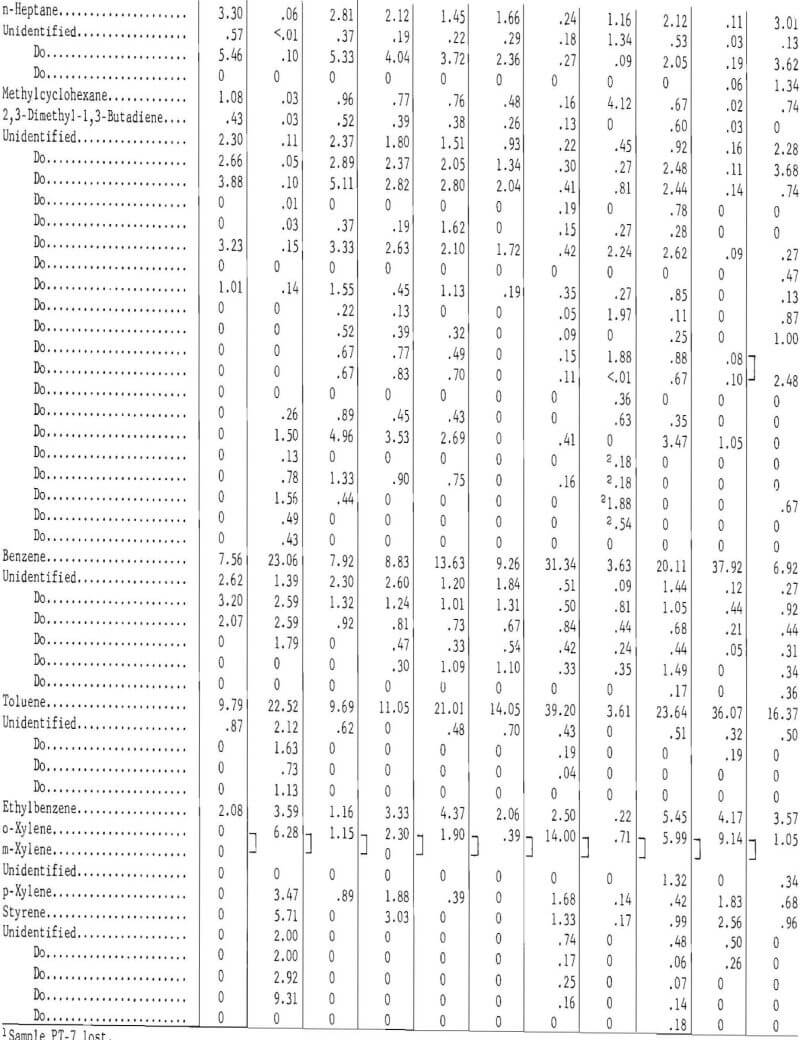
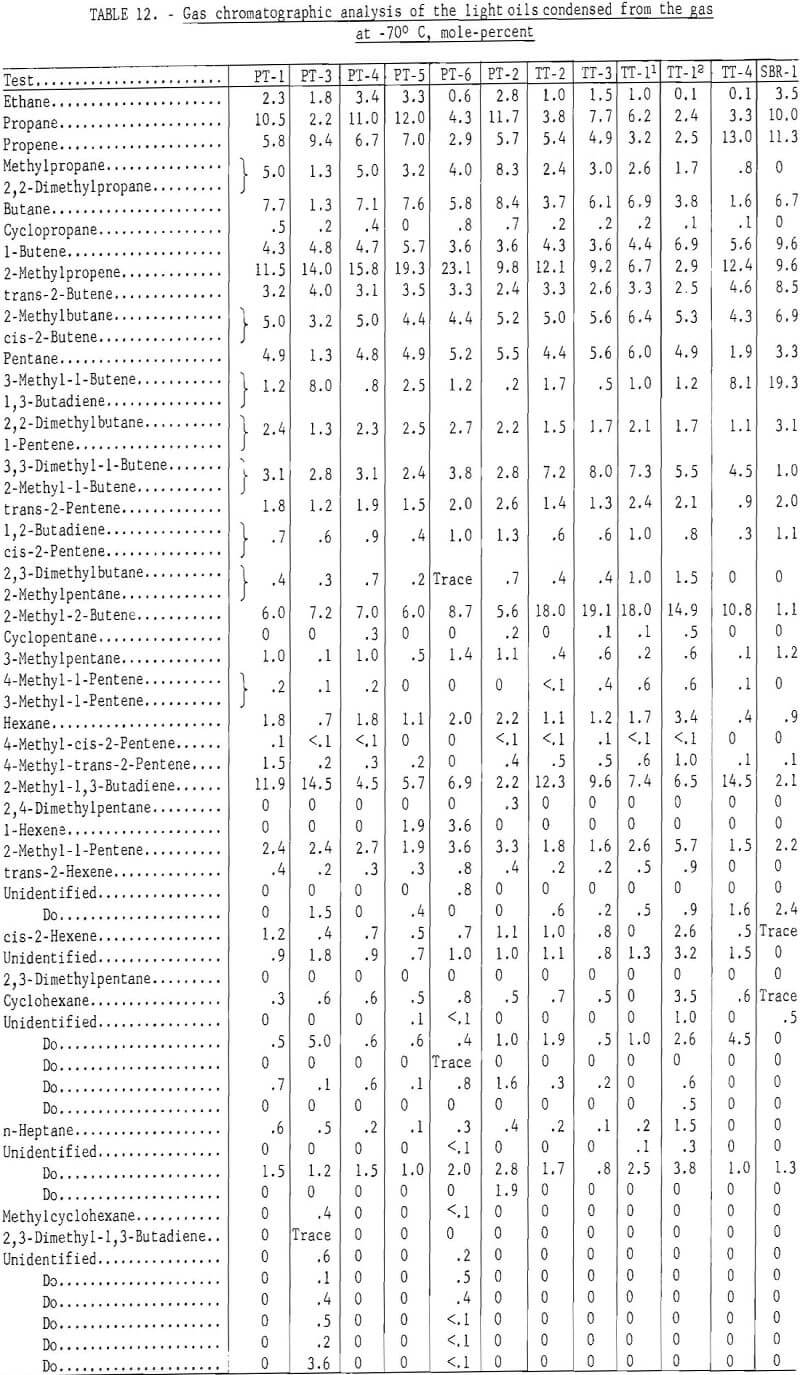
Prior to backflushing (table 9) 102 compounds were identified by mass spectrometry and internal standardization. Not all compounds were present in every sample and not all were completely resolved by gas chromatography. The mole-percent values follow the major constituent in the groups of compounds that were not completely separated (tables 9 and 11).
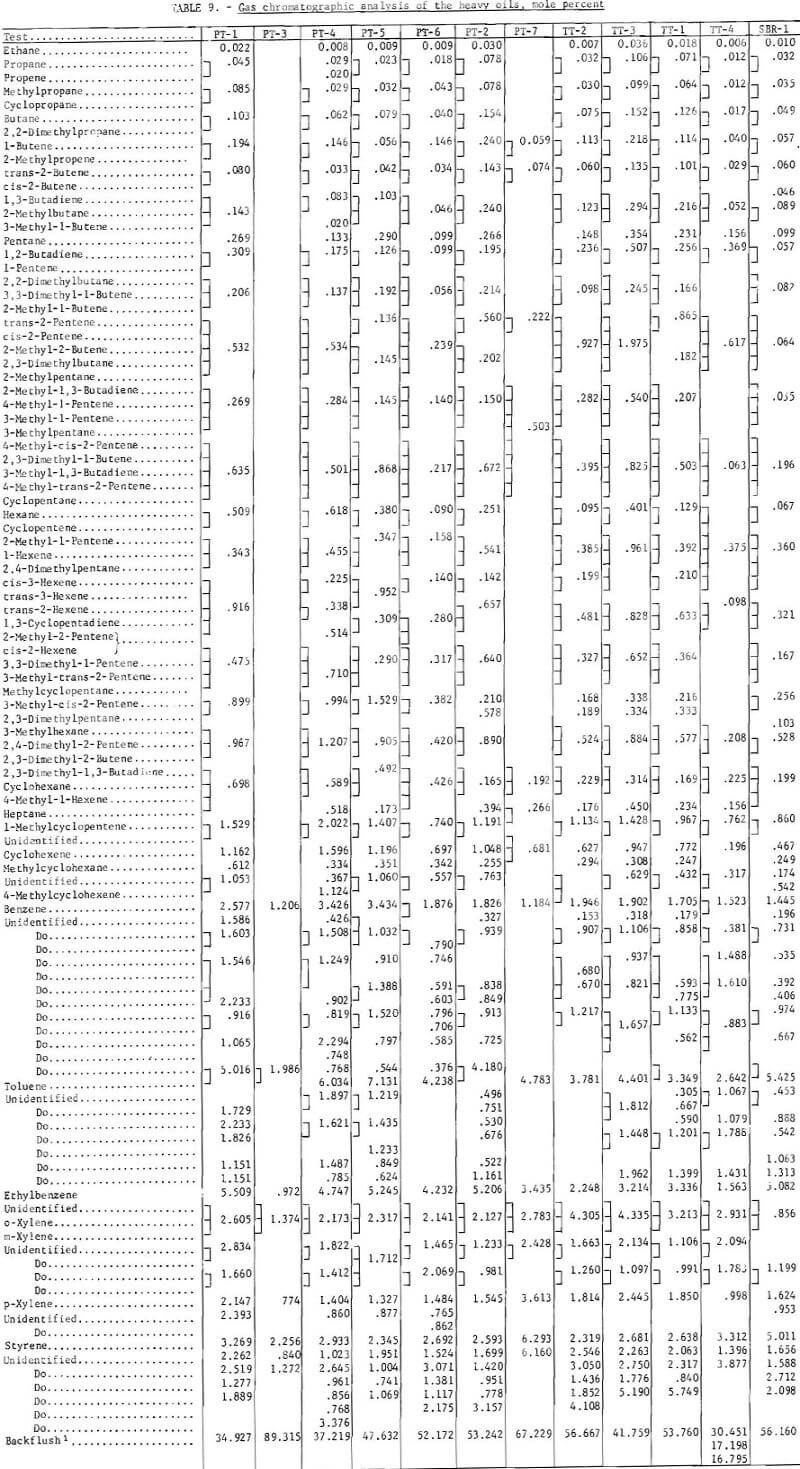
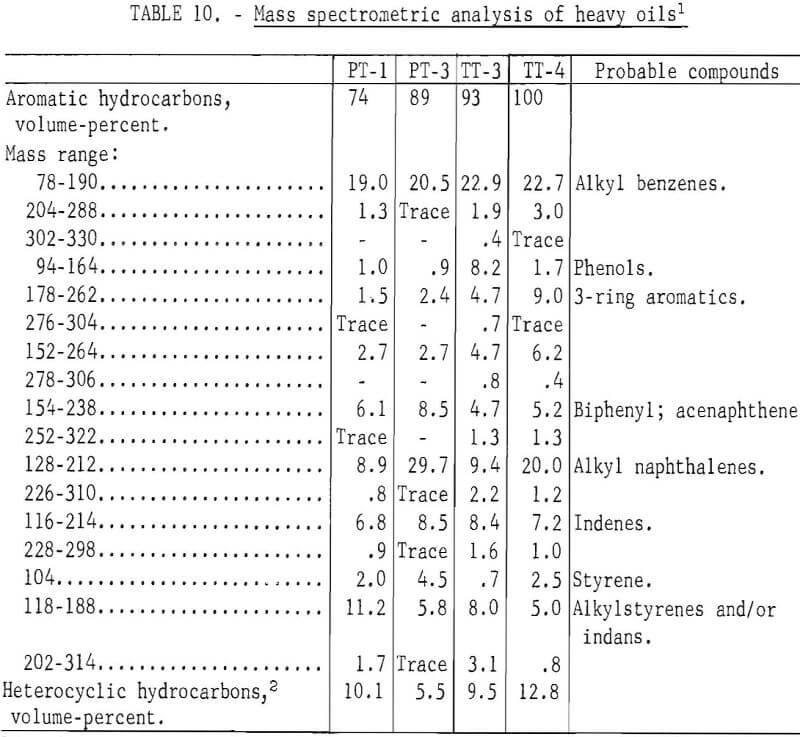
A mass spectrometric analysis of four of the samples are given in table 10. Alkyl benzenes in the 78-190 mass range and alkyl naphthenes in the 128-212 mass range were the predominant components of the heavy oils. The mass spectra obtained at low ionizing voltage indicate PT-3, carbonized at 900° C, is much more aromatic than PT-1, carbonized at 500° C, With the exception of alkyl benzenes, PT-3 has its maximum peak height at the molecular weight of the unsubstituted ring system; PT-maximum appears with the addition of 2-3 alkyl carbons in the ring system. Similar comparison can be made for TT-3, carbonized at 500° C, and TT-4, carbonized at 900° C. The alkylated ring systems indicate that TT-4 is much more aromatic than TT-3. The maximum molecular weight indicated by the mass spectra is appreciably higher for the TT (truck tire) samples, which contained more natural rubber than for the PT (passenger tire) samples, even though the major constituents are low molecular weight.
Light Oils
Table 11 gives the gas chromatographic analysis of the light oils condensed from the gas at 0° C, and table 12 the composition for the light oil condensed at -70° C. The following procedure was used to analyze the light oils. Components in the light oils that eluted prior to benzene were determined with a dual flame ionization chromatograph and were eluted on a 25-foot-long, ¼-inch-diameter column containing 10 percent dimethylsulfolane (DMS) on 45- to 60-mesh Chromosorb P. Benzene and higher molecular weight compounds were determined by the same procedure and apparatus used for the heavy oils (tricresyl phosphate column). Pertinent operating conditions for the DMS column were as follows:
Carrier and flow…………………………………………………..helium at 5.45 ml/minute.
Temperature of injection port………………………………………………..150° C.
Temperature at detector……………………………………………………….200° C.
Column temperature………………………………………………………………..0° C.
Sample size…………………………………………………………………………….0.5 µl
The gas chromatographic analyses of the light oils condensed from the gas at 0° C indicate the effect of carbonization temperature on its composition. Ninety-eight components were identified. For the passenger tire tests (PT-1, PT-2, and PT-5), carbonized at 500° C, about 70 percent was eluted prior to benzene, whereas at 900° C, only 5.17 percent was eluted. The latter light oil contained 23.06 mole-percent benzene and 22.52 mole-percent toluene. The light oil from the 500° C test (TT-3) on the truck tires eluted 89.59 per-cent prior to benzene, whereas at 900° C (TT-4) only 6.22 percent was eluted prior to benzene. The results for those tests run at or below 500° C in which the temperature was increased to increase gas yields are intermediate between the 500° and 900° C tests.
Sixty-one components were identified in the gas chromatographic analysis of the light oils condensed from the gas at -70° C. Most of the components were eluted prior to 2,4-dimethylpentane, with the oils from the lower temperature carbonization tests eluting more of the saturated and less of the unsaturated hydrocarbons than the higher temperature tests.