Table of Contents
Two methods for determining the oxygen content of cyanide solutions are offered as being simple and accurate. White’s method is a colorimetric one, depending on the degree of coloration imparted to a solution of pyrogallic acid in the presence of caustic soda. Weinig and Bowen’s method, a modification of that of Schutzenberger, depends on the reducing action of a sodium hydrosulphite solution on a solution of indigo blue (indigotin disulphonate).
White Method
This method was developed by H. A. White, consulting metallurgist for the Union Corporation of Johannesburg, and is based upon the color imparted to an alkaline solution on the addition of pyrogallic acid, the “pyro” used in photographic work.
APPARATUS REQUIRED
1 dozen 250-cc glass-stoppered bottles.
1 burette.
CHEMICALS REQUIRED
Sodium hydrate (NaOH) solution, 2N (80 grams per liter).
Pyrogallic acid or pyro (the crystalline salt is preferable to the powder).
Brown dye (Diamond brown or caramel).
PREPARATION OF STANDARD COLORS
Saturate a quantity of ordinary tap water with oxygen by passing air through it for an hour. Then stand for another hour to remove bubbles of occluded air. To one of the 250-cc bottles containing this water add about 1/10 gram of pyro and 1 cc 2N NaOH. The pyro crystals must immediately sink below the surface. Then insert a glass stopper with a twisting motion to exclude any small air bubbles. After the soda solution is added, the bottle should be filled to within ¼ in. of the top, so that the stopper may be inserted at a slight angle. Shake the bottle until all the pyro is dissolved.
The water will take on a reddish-brown color corresponding to the oxygen content of oxygen-saturated water at the existing temperature and pressure. The percentage oxygen content of water, saturated under any given set of conditions, may be calculated from the chart of Weinig and Bowen (Fig. 55). This color is then matched with a water solution of Diamond dye or caramel. A small amount of chromate of potash will sometimes assist in obtaining the exact shade.
Assume that under the existing conditions the saturation point of water corresponds to 8 milligrams oxygen per liter. Then if standard bottles are made up containing 1 part color corresponding to saturation and 7 parts water, this lighter color will correspond to 1 milligram oxygen per liter, and equal parts of standard color and water will correspond to 4 milligrams oxygen. In the same way make up a series of eight bottles, colors in which represent oxygen contents of from 1 to 8 milligrams oxygen per liter.
TESTING THE CYANIDE SOLUTION
Fill one of the 250-cc bottles with the solution to be tested. Use a rubber tube reaching to the bottom of the bottle, and avoid all agitation. A drop or two of kerosene oil in the bottle will film the solution and still further prevent absorption of oxygen. Fill the bottle nearly full, then add 1/10 gram pyro and 1 cc 2N NaOH, and stopper instantly, taking care that no air bubble is left under the stopper. Shake well, and compare with standard colors.
Some solutions, particularly those resulting from the cyanidation of silver ores, show fading colors and become cloudy on the addition of pyro and soda, according to E. M. Hamilton in E. and M.J., July 17, 1920. In such cases a better comparison is made after the solutions stand a definite time, say 3 to 6 min. Also, in such solutions a better standard color is obtained by making up with a regular plant solution to which is added the usual amount of soda and pyro. Then, after standing 3 to 6 min., this color is matched with the dye or caramel, and the fractional standards prepared as usual. A small amount of solid pigment such as yellow ocher, added to the dye or caramel solution, will match the precipitate which sometimes forms. With such solutions it is better to make up a fresh set of standards whenever oxygen tests are to be made.
Weinig-Bowen Method
The Weinig-Bowen method determines oxygen accurately to tenths of a milligram per liter of solution or 1 part oxygen in 10 million parts of a solution on a 250-cc solution sample, with a proportionately greater degree of accuracy on larger samples.
Reasonably clear mill solutions are best sampled by siphoning them through a rubber tube and glass tube into Winchester acid bottles. Pulps should be settled, and the clear liquor siphoned off.
A convenient quantity of standard sodium hydrosulphite solution is made up as follows: Fill a 2½-liter acid bottle with distilled water, preferably fresh. Dissolve in it 5 grams caustic soda, and then add 5 grams sodium hydrosulphite. Place a layer of kerosene over the solution. Then siphon the liquor into bottle b of Fig. 101. This solution deteriorates rapidly if exposed to the air, so, as shown in the insert a of the sketch, the cork (not rubber) is run in with shellac.
The indicator, indigotin disulphonate, is made up as follows: Place in a casserole 7 grams indigotin, and add 30 cc concentrated sulphuric acid. Place over a water bath, and heat to 90°C. for 1½ hr. or until all lumps disappear. Then dilute to 2 liters with distilled water. Neutralize the acidity by adding powdered limestone, a little at a time, allowing it to stand a few minutes between additions, until all action has ceased. Filter without washing, place in a corked bottle, and use as required. It is convenient to dilute this solution so that 1 cc of the indicator is equivalent to 0.25 milligram oxygen per liter solution. This will indicate 1 gram per liter when a 250-cc solution sample is taken for titration. This indicator does not deteriorate and may be kept in a well-stoppered bottle.
Figure 101 shows the apparatus for this test as follows: two 2½-liter acid bottles a and b, a 250-cc flask c, a 50-cc burette d with side connection, a common burette e, a clamp stand f to hold two burettes, a 400-cc beaker with 250-cc point scratched on it, a glass stirring rod, 3/16 in. glass or lead tubing and rubber tubing for connections, a pinchcock g for bottom of the rubber connection on the burette that contains standard hydrosulphite solution, and a container for kerosene to be used in the procedure. When setting up the apparatus, the relative positions of the parts shown should be closely observed.
The bottles are filled as follows:
Remove the connection x, and place a cork stopper in the top of the burette so that no solution can overflow. Place a bottle containing 2½, liters of kerosene so that its bottom is above the top of bottle a, and connect this bottle to the bottom of burette d with a siphon. Open pinchcock g and stopcock j, and allow kerosene to siphon into bottle b until filled. Replace the bottle that contained kerosene by a bottle containing the standard solution of hydrosulphite. This solution should always be covered by a layer of kerosene; siphon the standard solution into bottle, b, the kerosene being forced from bottle b over into bottle a automatically. As soon as the hydrosulphite solution has reached to within 1 or 2 in. of the top of bottle b, close both the pinchcock g and stopcock j. After flask c has been nearly filled with kerosene, place connection x in top of burette d, and seal with dry shellac dissolved in alcohol. Open stopcock j, keeping pinchcock g closed, and allow the standard solution to pass into burette d until it just enters flask c; then close stopcock j, open pinchcock g, and allow the standard solution to drain completely; its action as a siphon will draw the kerosene over into burette d. The standard solution is now drained off to eliminate any possibility of its being exposed to air and to give it a cover of kerosene in burette d. Close pinchcock g, open stopcock j, and allow burette d to fill to zero mark. The layer of kerosene prevents admission of air during this procedure.
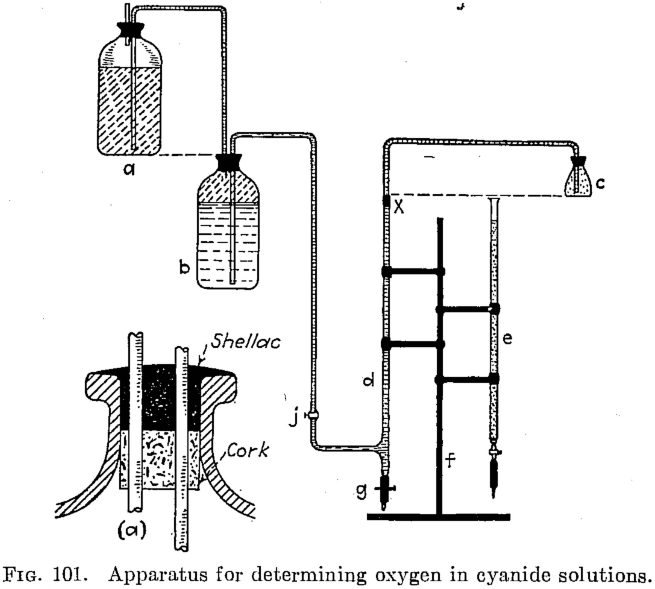
Then the apparatus is ready for use. Fill burette e with the indicator, and place a glass cover over the top to prevent evaporation.
Routine titrations are as follows: The solution sample is siphoned over beneath the kerosene into the 400-cc beaker to the 250-cc mark. Alkalinity is neutralized with dilute sulphuric acid, 1 cc or less of indigotin disulphonate is added as indicator, and titration is completed with the hydrosulphite. Then the necessary correction is made for the indicator, and the result is converted into milligrams of oxygen per liter of solution or percentage saturation, as may be desired. The end point in clear solutions is a slight yellow, but with others it may be white to gray, especially if certain salts are contained. The kerosene may be used several times by pouring the contents of the beaker into a large bottle, after titration, then siphoning off the kerosene for reuse after enough has accumulated. General circulating-plant cyanide solutions have 7 to 75 per cent maximum oxygen saturation. A number of precautions must be taken, but these will probably suggest themselves.
Determination of Reducing Power
To 5 to 25 cc of solution, depending upon the amount of reducing agents present, add sufficient water to bring the volume to 200 cc. Then add 25 cc of 25 per cent H2SO4 solution and titrate with 0.10N potassium permanganate (KMnO4) solution to the first faint pink coloration, which remains permanent for 2 min.
The result is reported in cubic centimeters of 0.10N KMnO4 solution per 1000 cc of cyanide solution.
The amount of cyanide solution taken for this determination should be so adjusted as to require 5 to 10 cc 0.10N KMnO4 solution. Larger titrations generally result in fading end points.
0. 10N KMnO4 Solution. Dissolve 3.16 grams of potassium permanganate in water, and dilute to 1000 cc. This solution should be kept in a dark bottle.
