Table of Contents
This excellent volumetric method commends itself as a means of estimating the fineness of silver—firstly, owing to the large number of possible impurities which do not affect its accuracy (these impurities include Cu—up to 70%— Au, Pb, Zn, Bi, Cd, Fe, Mn, Sb, and As) ; secondly, the method combines accuracy with speed.
The above impurities are the only ones likely to be present in the B. H. A. S. Pty, Ltd. refined silver, and, with the exception of Cu and Au, the others, if present at all, would be well under 0.1 parts per 1000.
Briefly stated, the method consists in the solution of the refined silver in HNO3, addition of ferric alum (indicator), and titration with an alkaline (either ammonium or potassium) thiocyanate solution until the brown ferric thiocyanate is formed, and gives a definite tint to the solution. The method is used at the Port Pirie smelters, and the outstanding feature in the method as there applied is the type of burette employed. This burette enables the operator to read 0.01 of a cc. with ease, whereas, with the ordinary burette fitted with a float, 0.05 of a cc., when a number of readings have to be taken, becomes very straining to the eyes. Moreover, waiting for the float to rise, and dislodging air bubbles from it, hinders rapid working. A description of the burette and fittings is appended with fig. 2.
DETAILS OF THE METHOD Volhard’s Method
Solutions required are:
(a) Stock Solutions.—
- Ammonium thiocyanate, 40 grm. per litre of water; 30 litres are prepared and placed in a carboy with glass syphon attached.
- Ferric alum, 250 grm. per litre ; two litres are prepared.
(b) Working Solutions.—
- Ammonium thiocyanate.—930 cc. of stock solution are syphoned off and made up to 5 litres with water. Thirty litres are prepared and placed in a carboy fitted with a glass syphon. The strength of this solution is tested immediately. One gram of standard silver is titrated, and should require 99.75 cc. or thereabouts. This ensures the refined silver titration being completed at about the middle of the capillary tube on the burette. Some slight adjustment of the solution may be required, but a little practice enables one to do this readily.
- Ferric alum (indicator).– One litre of stock solution added to 4 litres of water.
- Nitric acid.—Two litres HNO3 (1.4 sp. gr.) to 1.5 litres of water.
The working solution of ammonium thiocyanate is stirred well with a rod every day before using.
Standard Silver.— This has a fineness of 999.4, and is granulated.
 Standards.—Weigh out three lots of 1 grm. of standard silver; place in a 700-cc. flask (see Fig. 1). Add 5 cc. HN03 solution, place the flask on a
Standards.—Weigh out three lots of 1 grm. of standard silver; place in a 700-cc. flask (see Fig. 1). Add 5 cc. HN03 solution, place the flask on a
hot plate, and heat until all brown fumes are driven off and silver dissolved. Remove and allow to cool for at least 15 minutes, add 100 cc. of cold water and 5 cc. ferric alum indicator. The standards are now ready for titration.
Titration.—Fill the burette with the working solution of ammonium thiocyanate and run out; repeat this four or five times to ensure that the burette is clean. Fill the burette and remove all air bubbles by raising the jet of the valve and allowing a little of the solution to run out. Finally adjust the solution to the mark on the top capillary tube. Run the solution into the flask (keeping the flask agitated all the time) to the 99.75 mark. Remove all solution from the end of the burette by tapping gently three or four times on the neck of the flask, and rinse the neck of the flask with cold water from a wash bottle. Now shake the flask vigorously 200 times, again rinse the neck of the flask, and read the burette. The time taken for shaking (about 2 minutes) is sufficient to enable the solution in the burette to drain thoroughly.
A bracket at a convenient height, and long enough to take three flasks abreast, serves to hold the flasks when titration is completed. A piece of white paper is placed as background, and enables one to distinguish the brown tint readily. Three standards are done, and their colours compared. Two of those must agree before accepting one as the standard.
Precautions.—
- The wash water should be about the same amount for each assay, and as little as possible.
- The time allowed for draining of the solution in the burette should be sufficient.
Calculation.—
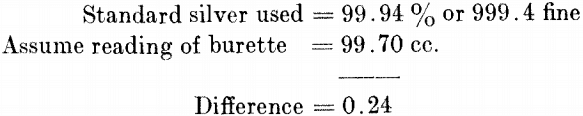
This shows the solution to be slightly stronger than 1 cc. = 0.01 grm. of silver, and that it is necessary to add 0.24 to the readings of the burette to obtain % of silver. Moving the decimal point one place to the right gives fineness of silver or parts per 1000.
Refined Silver.—Take 1 grm. of sample (granulated), place in a 700-cc. flask, and proceed in the same manner as for standard. Carefully add the last few tenths, so that the final brown tint obtained agrees with the tint produced in the standard. To the burette reading add 0.24 and move the decimal point one place to the right, which gives parts per 1000 or fineness of the sample.
Burette and Fittings.—That portion of Fig, 2 above A is of special construction, and might be termed the burette proper. The length of this portion is 1 ft. 2 in., and consists of the two capillary tubes AB and CD and the sphere E. The capacity of that portion of the burette B to C is exactly 99 cc. B to A holds the remaining cubic centimeter, and is the only part which is graduated. It is divided into tenths, and these again into tenths, thus marking one-hundredth of a cc. One-tenth of a cc. in this capillary tube lowers the column 0.7 of an inch, so that the one-hundredth divisions are about 0.07 in. apart, and are thus easily read off.
Below A are the fittings, which consist of rubber tubing F, T-piece K, taps L and M, glass valve, and jet O. One end of the T-piece communicates to the, working solution of ammonium thiocyanate, the supply of which is controlled by taps L and M. To the other end of
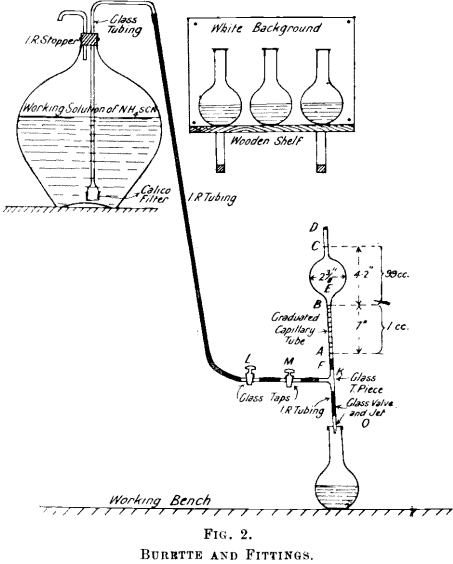
the T-piece is fixed the glass valve, which consists of a piece of good rubber tubing into which is fitted tightly a piece of glass rod about ½-in. long. The ends of the glass rod are tapered slightly and rounded off. To the other end of this rubber tubing is attached the jet, which is similar to the ordinary wash-bottle jet. A gentle pressure on the glass rod which is in the rubber tubing allows the solution to run out.
