The corrosion of black rolled mild steel in sodium cyanide solution is negligible. A piece of steel suspended in a sodium cyanide solution maintained at 0.05% NaCN and 0.001% CaO, in the presence of air, lost 0.002% of its weight in two weeks, this was equivalent to a penetration of 0.76 micron per year.Oxidized iron minerals such as hematite, magnetite, goethite, siderite and the silicate iron minerals are practically unaffected by cyanide solutions. Complex iron carbonate minerals, such as ankerite, are said to decompose to some extent in cyanide solutions of low alkalinity (below pH 10) forming ferrocyanides. The sulphide iron minerals decompose appreciably in cyanide solutions, however, the extent of decomposition depending on the particular sulphide mineral, the particle size and solution conditions.
The three most important iron minerals in cyanidation are pyrite, marcasite and pyrrhotite. These minerals decompose in cyanide solutions in the order given, i.e., pyrite is the most stable and pyrrhotite the least. Pyrite, FeS2, is insoluble in hydrochloric acid, but decomposes readily in nitric acid, most of the sulphur going to sulphuric acid. Marcasite has the same chemical formula as pyrite but a different crystalline form. It tarnishes and decomposes much more readily than pyrite. Hydrochloric acid has practically no effect on it. Nitric acid decomposes marcasite readily, most of the sulphur separating in the free state; in this respect it differs from pyrite. Pyrrhotite varies in chemical composition from Fe5S6 to Fe16S17; it is said to be ferrous sulphide, FeS, containing various amounts of dissolved sulphur. Pyrrhotite dissolves in dilute hydrochloric acid evolving hydrogen sulphide and leaving elemental sulphur, e.g.:
Fe7S8 + 14 HCl = 7 FeCl2 + 7 H2S + S
Sometimes iron sulphide minerals are intimately associated with minerals such as melanterite, FeSO4*7 H2O. This mineral is water-soluble and reacts readily with cyanide.
The decomposition of iron sulphides in water, in alkalis, and in cyanide solutions has been the subject of much investigation and speculation. More fundamental research work has been done on the decomposition of pyrite in various solutions than either marcasite or pyrrhotite. The latter minerals have been studied more in actual precious metal ores than in simple systems consisting of a pure mineral and a solution. Inasmuch as pyrrhotite is frequently associated with other sulphide minerals in precious metal ores such as arsenopyrite, chalcocite, etc., some doubt may exist as to whether a certain effect in cyanidation can be attributed to the pyrrhotite solely.
When 100 grams of minus 200 mesh high grade pyrite and 30 ml. of distilled water were agitated on rolls for 25 minutes and filtered, the filtrate was found to contain 0.736 grm. of ferrous sulphate. A second treatment of the same pyrite with fresh distilled water showed the filtrate to contain 0.030 grm. of ferrous sulphate, while a third treatment produced only 0.009 grm. of ferrous sulphate. The amount of sulphate ion in solution was more than the equivalent of all the iron in solution. This indicated that some of the ferrous sulphate was hydrolyzed to sulphuric acid and ferrous hydroxide. The latter was believed to be adsorbed as a coating on the pyrite surface which retarded further decomposition of the pyrite. Similar experiments conducted on pyrite of various screen sizes showed that the amount of ferrous sulphate found in solution was a function of the surface of pyrite exposed.
As a result of the action of lime and air on a pyritic flotation pulp soluble sulphides, thiosulphates and minor amounts of colloidal sulphur were formed. Dissolved oxygen and soluble sulphides reacted to produce thiosulphate and sulphate. In the presence of pyrite, dissolved oxygen and thiosulphate interacted with the formation of sulphate. In the absence of pyrite, calcium thiosulphate solutions remained unchanged through aeration. When made alkaline with lime they also remained unchanged, but when they were acidified, they decomposed. The results of prolonged oxygenation of fine pyrite in lime solution showed that causticity dropped rapidly at the beginning and more slowly later; the maximum amounts of the soluble reducing agents, calcium sulphide and calcium thiosulphate, were formed as the causticity approached zero (pH 8). Apparently, the presence of calcium hydroxide was necessary for the formation of soluble sulphides. Below pH 8, reducing agents rapidly disappeared and the only product formed by the action of oxygen on the pyrite was sulphate, mainly sulphuric acid.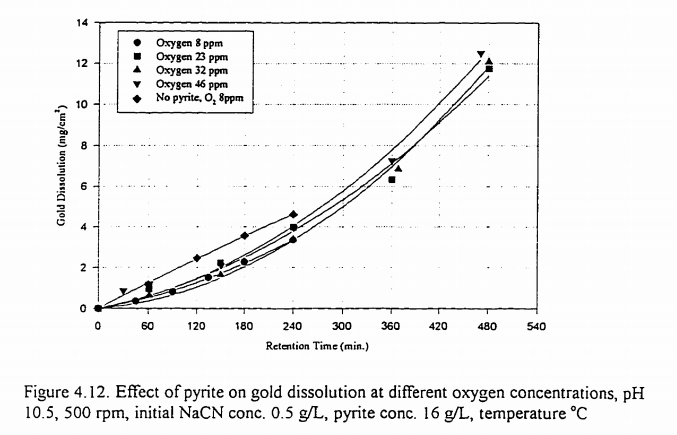
The following procedure was adopted to investigate the reactions taking place in a pyrite, sodium hydroxide and air system: High grade pyrite was dry crushed to pass a 150 mesh screen. Twenty gram portions of pyrite were transferred to separatory funnels containing 400 ml. of alkaline solution. Air was passed at a constant rate up through the stems of the funnels. Samples of the pulps were taken at various intervals, filtered and analyzed. One solution of pH 10.8 at the start, dropped to pH 3.2 after 30 hours aeration; another solution dropped from pH 12.1 to pH 8.5 in the same period. The products of the reaction of air on alkaline pyrite pulp were found to be sulphide, thiosulphate, sulphite, sulphate, and small amounts of free sulphur. Much more sulphide was found in the solution started at pH 12.1 than in that solution started at pH 10.8. The sulphide and sulphite content of both solutions decreased as the alkalinity decreased. The sulphate, thiosulphate and total sulphur gradually increased with time.
The sulphide is oxidized while the hydroxyl ions are retained or adsorbed on the surface thereby inhibiting any further reaction. However, if the circuit becomes too acid, the surface hydroxyl ions would be removed and fresh surfaces exposed for further decomposition.
Further experiments were carried out in which the effects of cyanide on pyrite were investigated; the procedure was similar to that previously described. Preliminary tests, run in the absence of pyrite showed that sodium thiosulphate and sodium cyanide, in the presence of air, react very slowly to form thiocyanate; under similar conditions sulphur and cyanide react much more rapidly to form thiocyanate. In the absence of air, sodium sulphide and cyanide react very slowly to form thiocyanate; with air present this reaction is much more rapid but slower than the reaction between sulphur and cyanide. With pyrite present, the formation of thiocyanate proceeded continuously. This accounted, however, for only about one third of the total loss of cyanide. At least some of the remaining loss was believed to be caused by fixation of cyanide on the pyrite. Conditioning the pyrite in an alkaline solution caused a pronounced reduction in the amount of cyanide lost.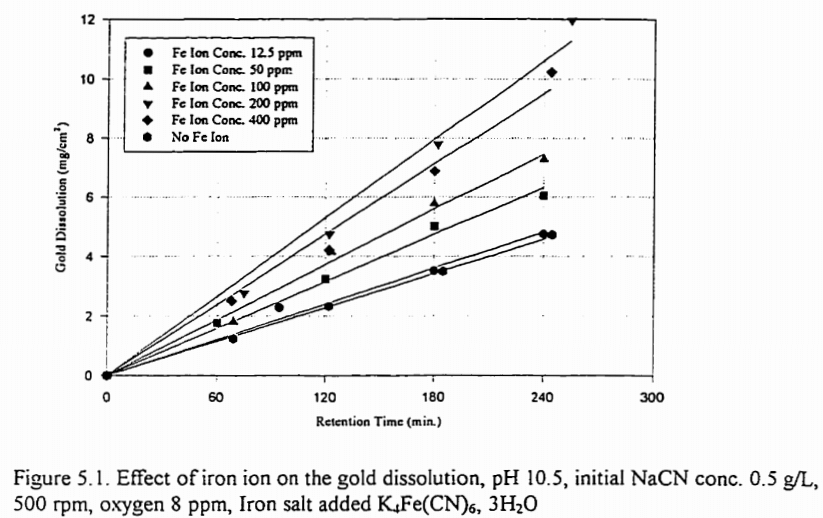
An EXAMPLE Cyanidation Practice is described in the treatment of a high pyrrhotite ore that is essentially a complex quartz, carbonate, sulphide ore having the following approximate composition: SiO, 19.6%; Fe 32.2%; S 14.6%; As 3.5%; Al2O3 0.4% ; MnO 0.9%; CaO 2.4%; MgO 7.6%.; Cu 0.1%.; CO, 15.7%% Au 0.60 oz/tonne. It consists of a finely crystalline admixture of quartz, calcite, dolomite, siderite, ankerite, pyrrhotite, pyrite and arsenopyrite as the principal minerals, with small amounts of chalcopyrite and chlorite; pyrrhotite is the predominating sulphide. The gold is most generally associated with arsenopyrite in contact with pyrrhotite. The general rule is that highly arsenical ore is rich in gold, while highly pyrrhotitical ore is relatively poor.
In treating this ore, a high gold product is concentrated on tables and strakes; this concentrate is treated separately for the recovery of gold. The strake tailings, with 0.5 lb. of lime per ton added, are ground and separated into sands and slimes which are cyanided separately. Solutions from cyanidation are high in ferrocyanide and thiocyanate due to pyrrhotite. If the solutions are reused for further dissolution of gold and silver, these impurities build up so rapidly in solution, to such high percentages, that precipitation of values becomes not only a problem, but almost an impossibility. Consequently, solution volumes must be kept low, and cyanidation in thick pulp reverted to. Barren solutions are discarded.
Each charge of sand or slime is agitated and aerated for 12 hours when solution of the gold appears to attain equilibrium. Starting at 100 grains (0.208 oz.) per ton, the assay of the sand comes down to 25 to 30 grains (0.052 to 0.063 oz.) per ton after which further agitation gives but slightly increased solution of the gold. Prolonged agitation is not profitable, as cyanide solution strength falls rapidly and reprecipitation of gold may take place. Commencing at about 0.25% NaCN for sands or slimes, cyanide strength falls to about 0.08% NaCN for sand charges, and 0.02% NaCN for pure slime charges, with about equal extraction of gold in each case. Hence, the overproduction of slime increases cyanide consumption. About 0.5 lb. of solid lime per ton of sand is added with the cyanide. The addition of 3 to 4 lbs. of lime per ton maintains higher cyanide strength but raises the tailing values to 50 grains (0.104 oz.) per ton. Best results appear to be obtained with low alkalinities, the addition of much lime only tending to the formation of calcium polysulphides. A partial analysis of a typical pregnant solution going to zinc boxes shows it to contain 0.012% free NaCN, no protective alkalinity, 0.182% Na4Fe(CN)6, trace Na3Fe(CN)6, 0. 066%’ NaCNS, 0.005% copper, 0.0005% arsenic.
Further cyanidation developments along the lines of alkalinity control are described as excess lime in the cyanide pulp was known to be harmful. For maximum gold extraction, the optimum lime demand was small. The usual method for titrating alkalinity was found to be of doubtful value when the alkalinity was very low, particularly in the presence of some dissolved salts having buffering properties. Therefore, pH notation was used as the measure of alkalinity. It was found that when cyaniding a pulp of high density, the optimum pH value of the solution lay between 8 and 9. When using solutions only once, it was found difficult to maintain the pH within these limits. A barren solution was found much better in this respect. At alkali levels above pH 10, cyanide consumption and dissolution of gold decreased; cyanide destruction tended more towards the formation of thiocyanates, seeming to confirm the hypothesis of the attack of excess alkali on the pyrrhotite. Below pH 7, cyanide destruction was very great, the tendency being rather towards the formation of ferrocyanides and the liberation of HCN gas.
The use of various metallic salts was investigated. Cadmium and zinc salts modified cyanide consumption, apparently by suppressing ferrocyanide formation, while lead and mercury salts, in addition to reducing cyanide consumption, permitted a greater extraction of gold. Per unit weight of reagent mercuric salts were more active than mercurous salts while both were more active than lead. Among the lead salts tested, the soluble salts were more active for the same weight of metal than the relatively insoluble oxides. As the quantity of lead was increased so did the relative proportion of the thiocyanate increase over the ferrocyanide in the final solution, while total cyanide consumption was reduced. In the presence of lead, the pH of the cyanide solution at the start was 9.6, dropping to pH 8 when maximum gold extraction was obtained.
With optimum amounts of lead and other reagents, the speed with which the gold was dissolved was roughly proportional to the quantity of air injected. However, the high cost, and the unstable pH conditions induced in the solution by intense aeration of the pulp outweighed the advantages of increased speed of gold dissolution.
As a result of the foregoing findings cyanidation practice was modified as follows: The ore pulp, both sand and slime products, is fed to the cyanidation section as a thick, viscous pulp containing about 65 or 70% solids. While being run into stirrer tanks, a suspension of solid lime in water is added. Sand charges demand 0.7 to 1.1 lb. of lime per ton of solid, and slime charges between 2 and 3 lbs. per ton. Apparently the smaller the particle size, i.e. the greater the surface area, the greater is the lime demand of the pulp. The thick pulp is stirred and aerated for a short time. The pH of the solution during this preliminary conditioning is between 10.0 and 10.4. Barren solution is then added to the pulp to dilute it to 50% solids. At the same time, about 1 lb. of lead salt per ton of solids, dissolved in water, is added. The alkalinity of the solution is now pH 9.6 to 9.8 and it seems to be important that this upper limit should not be exceeded. Fresh sodium cyanide solution is added to the pulp to raise the free cyanide content of the solution to 0.07% NaCN. The pulp is stirred for 6 hours* At the end of this period, the pH of the solution is about 8. The pulp is filtered after the addition of a small amount of lime to aid filtration. Precipitation of the gold is carried out in zinc boxes. The present method of treatment extracts about 80% of the gold in the cyanidation feed; it has lowered the main tailing assay by approximately 50%.
Specimens of various rocks obtained from the mine were ground and cyanided in closed bottles with 0.1% NaCN solution. A specimen known to contain much ankerite, CaC03(Mg, Fe, Mn)C03, almost free from sulphides consumed a great deal of cyanide, forming ferrocyanide exclusively. No noticeable reaction was noted between siderite and cyanide if the pH of the solution were kept above 7.
