Solvent extraction as a method of purifying and concentrating metal values from solution has become a standard hydrometallurgical operation for recovery of both uranium and vanadium, and within the past two years it has been put into operation in a commercial plant for recovery of copper from leach solutions.
In this first full scale solvent extraction plant for recovery of copper operated by Ranchers Exploration and Development Corporation near Miami, Arizona, a kerosene solution of an organic compound, LIX-64, is used to selectively extract the copper from the dilute acidic copper sulfate leach liquor obtained from heap leach operations. The copper is back extracted from the solvent into a stripping solution which is fed to an electrolysis circuit producing cathode copper for sale.
Centrifugal force is a possible means of improving the speed with which the two liquid phases can be separated from each other. Unfortunately, for the capacities involved, centrifuges would be very expensive and although the solvent inventory could be significantly-reduced by performing the separation with centrifugal machines, the capital cost would remain high.
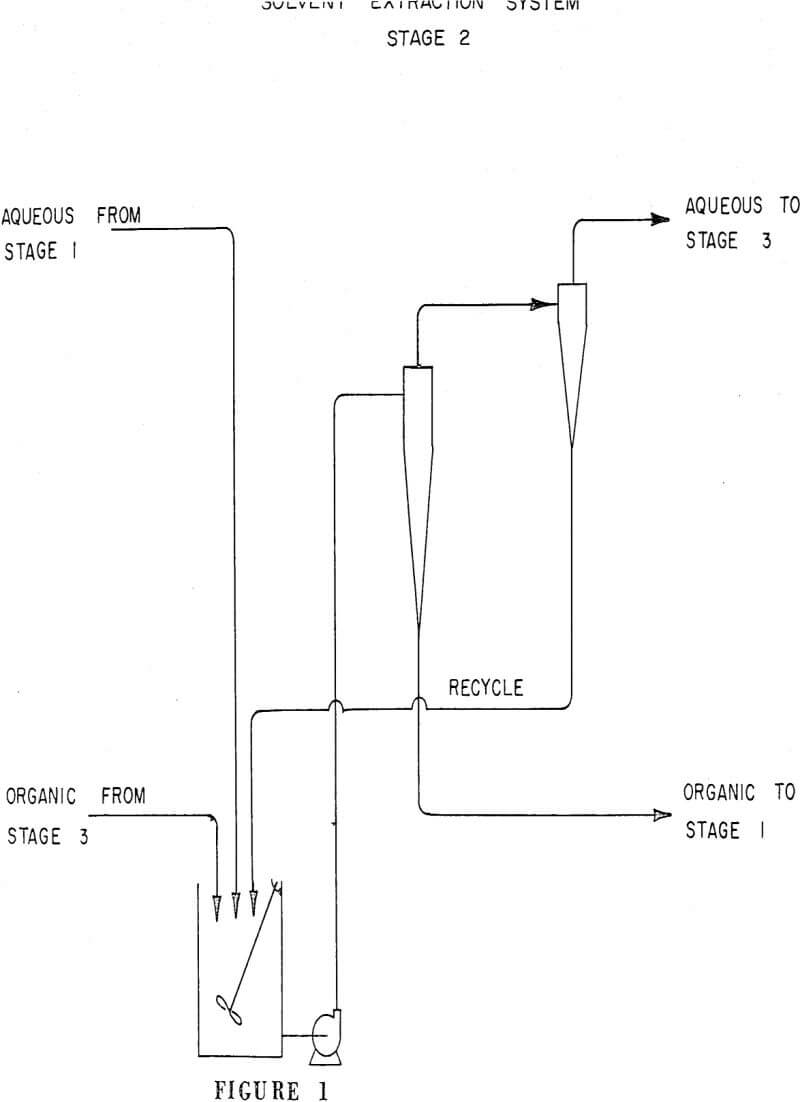
The separation of immiscible liquids by cyclones is considered to be more difficult than the separation of solid from liquid because density differences are usually smaller, and the existence of large shear forces can cause breakup and emulsification rather than coalescence of droplets of the dispersed phase.
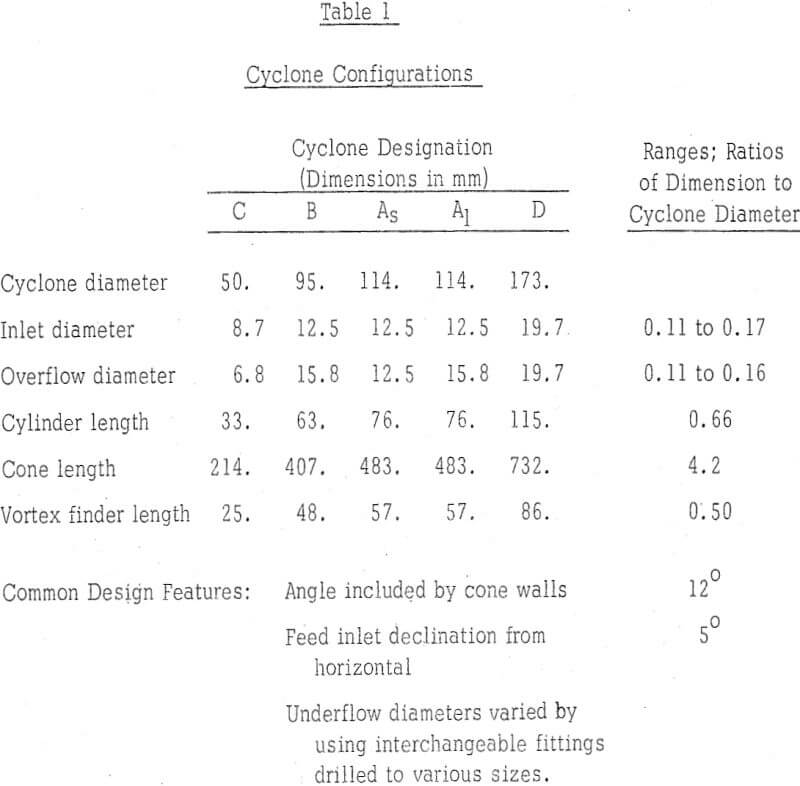
Kerosene in refined form is the usual diluent for reagents used in the extraction of copper from aqueous solutions. However, the small density difference between kerosene and water and the relative ease with which emulsions form between the two liquids lead to the consideration of other liquids as the solvent carrier. Perchloroethylene (tetracholorethylene) was the initial selection.
To obtain basic information on each cyclone, aqueous-organic dispersions were pumped from a tank through a 3,600 rpm centrifugal pump to the cyclone with overflow and underflow products returning to the tank. Timed overflow and underflow samples were simultaneously taken and the volumes of the phases recorded.
The volume split from a cyclone is largely controlled by the size of the underflow orifice. In addition, increases in pressure cause less volume to report to the underflow and the effect is observable.
The considerable difference in flow rate for the same size underflow orifice for a circuit operating aqueous continuous or organic continuous. Thus, at 35 psi the same cyclone processes only 83% as much organic continuous mixture as aqueous continuous mixture.

