Table of Contents
Previous research by the U.S. Bureau of Mines showed that autoclave cyanide leaching of used automobile exhaust catalysts for recovery of platinum group metals (PGM) was technically feasible. This work involves a more detailed investigation of the chemistry of the dissolution of PGM in cyanide solutions. The PGM species in solution were identified by ultraviolet spectroscopy. The Pt and Pd cyanide complexes were square planar in geometry, while the Rh complex was octahedral. The rate of dissolution of the PGM was also investigated using a rotating disk technique. Dissolution rates increased in the order of Rh, Pt, and Pd, respectively.
The U.S. Bureau of Mines has developed a process for the treatment of spent automobile exhaust catalysts for recovery of the platinum group metals (PGM) contained therein (Desmond, et al., 1991; Kuczynski, et al., 1992; Atkinson, et al., 1989). (All references to catalyst in this manuscript should be taken as automobile exhaust catalysts unless otherwise noted.) The process involves leaching at elevated temperatures and pressures with cyanide. The process has been successfully demonstrated on a laboratory scale, and has recently been scaled up to a pilot plant system at the Reno Research Center of the Bureau of Mines. However, the basic chemistry of the process has not been investigated in detail up to this point. The purpose of this publication is to discuss the chemistry of PGM leaching at elevated temperatures in a cyanide system.
It has been estimated that in the western world, 2.2 million tr oz of PGM were used in the manufacture of automobile exhaust catalysts in 1991 (Coombes, 1992). This included 1.57 million oz of Pt or 34 pct of total Pt demand, 370,000 oz of Pd or 8 pct of demand, and 302,000 oz of Rh or 83 pct of demand. The Stillwater mine in southwestern Montana is the only mine operating in the United States for the primary production of Pt, Pd, and Rh. The great majority of these metals comes from South Africa and the Russian republics. Recycling accounts for a small part of the PGM supply in the United States, and much of the catalyst collected in the United States is currently shipped overseas for processing. Development of a simple process to recover the PGM from catalysts would benefit the United States greatly by encouraging domestic recycling of these valuable and strategic metals.
Two types of catalytic converters have typically been manufactured for use in automobile exhaust cleaning. In older converters, Pt and Pd were added to catalyze the oxidation of carbon monoxide and unburned hydrocarbons. Newer converters also contain Rh, which catalyzes the reduction of oxides of nitrogen. The older converters are termed two-way or oxidation catalysts, while the newer converters are called three-way catalysts, as three major components of the exhaust gas stream are treated. Another difference in catalysts is the substrate that the PGM are deposited upon. The monolith type catalyst consists of a ceramic honeycomb structure composed of cordierite, a silicate-based material. A wash coat of high surface area alumina is applied to the cordierite and impregnated with the PGM. The pellet type catalyst, common in early converters but being used less frequently today, is composed entirely of high surface area alumina onto which the PGM is impregnated. Due to the different substrates, PGM loadings, and conditions while in service, every used catalyst sample is different. Often, the catalyst has been sintered or poisoned while in use. Careful sampling and analysis of used catalysts is, therefore, necessary.
There are several alternatives for the processing of automobile exhaust catalysts. However, many of these processes have never been taken beyond laboratory or pilot plant scale studies. Hoffmann (1988) has reviewed and critiqued several processing methods. The main methods currently in use are acid dissolution and plasma melting. Both of these methods have their drawbacks. For the acid dissolution process, waste disposal is a serious concern due to both the waste acid and the large amount of solubilized aluminum. In plasma melting, and indeed in all thermal processing methods, exhaust gas cleaning is a concern, especially with the lead contamination often present in used catalysts. A more selective hydrometallurgical process for catalyst treatment would be beneficial in decreasing waste disposal concerns, while at the same time avoiding the lead emission problem associated with high temperature processes.
The optimum leaching temperature in the USBM process was found to be 160° C. Above that temperature Pd recovery fell off significantly. No gases were introduced into the autoclave, so the pressure at 160° C was entirely due to steam pressure, which is about 90 psi at 160° C. Autoclave leaching was conducted under unstirred conditions. The monolith catalyst was crushed to minus 10 mesh and the pellet catalyst was treated as is, at about 1/8 in. in size. While this may seem quite coarse, the PGM are present on the surface of the catalyst, and therefore should be accessible to the leach solution even at these particle sizes. Reagent additions were 1 pct sodium cyanide and 0.1M sodium hydroxide. Under these conditions, recoveries of PGM from spent catalysts were usually at least 85 pct Pt and Pd, and 75 pct Rh, and often higher. Since different batches of catalyst showed very wide ranges of PGM content and physical condition, the recovery varied somewhat depending on catalyst type and history.
Platinum Leaching IN Laboratory
Because of the variable nature of used PGM bearing automobile exhaust catalysts, leaching rate tests were conducted on samples of pure elemental Pt, Pd, and Rh. Samples were obtained as powders and foils. The powders are very finely divided and appear black, and are thus called blacks. The blacks were obtained from the Aldrich Chemical Company and were at least 99.99 pct pure. Metal foil samples were at least 99.9 pct pure. For leaching tests conducted with automobile exhaust catalysts, a sample of monolith 3-way catalyst received from Johnson Matthey was used. This material was virgin reject catalyst, meaning that the catalyst was rejected during the manufacturing process and thus never saw service. The catalyst contained about 50 tr oz/st Pt, negligible Pd, and 10 tr oz/st Rh. Other chemicals used in this study were of reagent grade. Reverse osmosis water was used in all experiments.
Autoclave leaching was conducted in a 250-mL teflon-lined Berghoff autoclave. Typically, 50 g of catalyst was leached with 100 mL of solution containing 1 pet sodium cyanide and 0.1M sodium hydroxide for 1 h at 160° C. Due to the high surface area of the catalyst and the presence of the PGM on the surface of the catalyst, leaching was conducted at fairly coarse particle sizes of minus 10 mesh with no agitation. After the time at temperature had elapsed, the autoclave was cooled, opened, and the pulp filtered and washed. For leaching of PGM black samples, 0.1 g of material was accurately weighed out and leached with 100 mL of solution as with catalysts. Due to the small amount and fine size of the black samples, these tests were run under agitated conditions using a magnetic stir bar. All solutions were assayed for PGM content by inductively coupled plasma atomic emission spectroscopy.
Many transition metal complexes show characteristic ultraviolet (UV) spectra due to electronic transitions. Therefore, UV spectroscopy was used to identify the PGM species in solution. Although UV spectra usually are not detailed enough to provide “fingerprint” identification of a certain complex, the technique can be used as confirming evidence based on the previous history of the solution. Ultraviolet spectra were obtained with a Hitachi UV-Visible spectrometer. Optical glass cells with a path length of 1 cm were used.
Leaching rate tests were run using a rotating disk technique. This technique was applicable only with Pd and Pt, because the dissolution rate of Rh was too low to be measured with this method. In this approach, a disk of metal is implanted into the face of an inert cylinder, which is rotated at a given speed while submerged in the leaching solution. Other leaching rate tests were run using samples of PGM foils and blacks. Foil samples were placed in solution at time zero and removed after a suitable interval had passed. These samples were run under unstirred conditions, due to difficulties in reproducing the agitation level. The leaching rate was calculated from the solution PGM content, the leaching time, and the geometrical area of the sample. Samples of PGM blacks were tested in a similar fashion, except that the particles were fully suspended in the solution by agitation with a stir bar. Intermediate solution samples were taken to get a better indication of the time dependence of the leaching rate. The surface areas of the blacks were measured by the BET method, using nitrogen adsorption.
Results and Discussion
Hydrolysis of Cyanide
A significant factor that comes into play when high temperature cyanide leaching is utilized is the loss of cyanide by hydrolysis. The rate of the hydrolysis reaction is negligible at ambient temperatures but becomes significant above 100° C.
As a cyanide solution is heated, the rate of the following hydrolysis reaction increases rapidly:
CN- + 2H2O = HCOO- + NH3…………………………………(A)
Reaction A is pseudo-first order due to the high and essentially constant concentration of water in an aqueous solution. Therefore, the half life of the cyanide depends on temperature alone. The temperature dependence of the rate of the hydrolysis reaction has been published (Tan and Teo, 1987), and from that data, the half life at various temperatures can be calculated. At 20° C, the hydrolysis reaction is very slow, with a half life of 3.5 yr. At 100° C the half life is 8.1 h, while at 160° C it is 7 min. In the laboratory autoclave leaching studies, it generally took the autoclave about 1 h to heat up to temperature. By that time, 90 pct of the cyanide had been hydrolyzed. There is no advantage in long leach times at 160° C, as the cyanide will have effectively been decreased to less than 1/1,000 of its initial concentration in 10 half lives (70 min).
An additional complication of the hydrolysis of cyanide is that the products of the reaction, ammonia and formate, may react with PGM in solution. Ammonia can act as a ligand for transition metals, and formate may react to form carbonyl complexes, or possibly react as a reductant for the PGM. This increases the number of possible PGM species dramatically. Since the chemistry of the PGM depends on the species present, it was therefore necessary that the PGM species be identified and characterized.
Characterization of PGM Species
One of the major questions concerning the leaching of PGM in a cyanide system was the speciation of the PGM in solution. The most promising experimental approach for identification of the PGM species was UV spectroscopy. This is because many transition metal complexes show characteristic UV spectra due to electronic transitions. Sharpe has written a treatise (1976) on the cyanide complexes of transition metals. The most common complexes that would be expected under the conditions used in the catalyst leaching process would be tetracyanoplatinate(II), [Pt (CN)4]², tetracyanopalladate(II), [Pd(CN)4]², and hexacyanorhodate(III), (Rh(CN6)³-. The Pt and Pd complexes have square planar geometries, while the Rh complex is octahedral. The potassium salts of these cyanide complexes were obtained for use as standards in the UV analysis. Solutions of the PGM complexes were also generated by leaching samples of pure, elemental Pt, Pd, and Rh blacks under identical conditions as the catalyst samples, so as to form the same species in solution. The spectra of the leach solution from the blacks were then compared with the spectra from a solution containing the PGM standards.
For the Pt and Pd species, the results were fairly clear-cut. The spectra of the Pt species is shown in figure 1. It can be seen that the two spectra are very similar, thus demonstrating that the relevant Pt species is indeed tetracyanoplatinate(II). The UV spectra of the Pd species is shown in figure 2. As with Pt, the match with the standard is good, confirming the presence of the
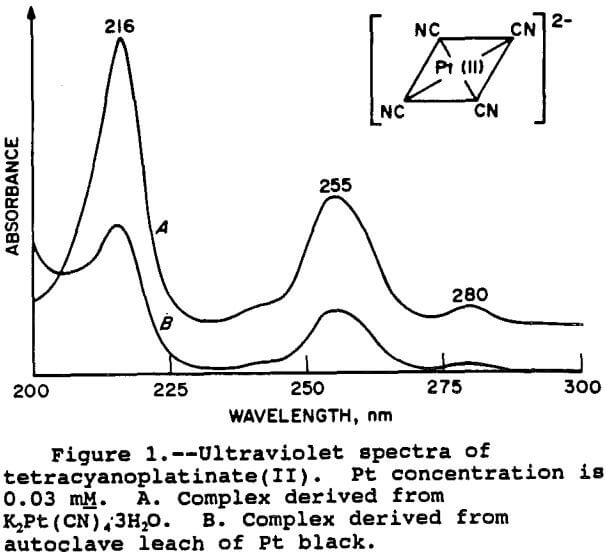
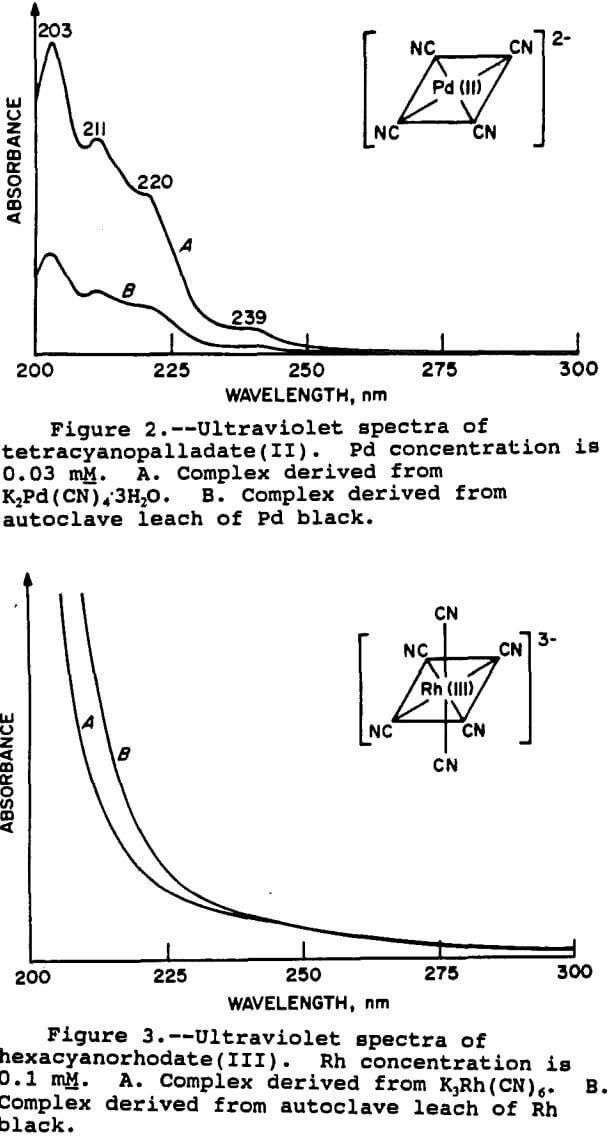
tetracyanopalladate(II) species. The wavelengths of the peak maxima also correspond with those given by Sharpe (1976) for both Pt and Pd.
For the Rh complex, the situation was different. The spectra for the Rh black leach solution and the hexacyanorhodate(III) standard are shown in figure 3. The spectra are not as distinctive as those of the Pt and Pd complexes. Although the spectra appeared to be similar, the evidence was not conclusive that the Rh was indeed in this form.
Therefore, additional samples of Rh complexes were examined. These included the ammonia complex, [Rh(NH3)5Cl]Cl2, the nitrite complex K3Rh(NO2)6, the acetate complex [Rh(CH3COO)2]2, and the chloride complex RhCl3xH2O. Each of these compounds gave UV spectra with distinctive peaks which did not agree with the leach solution spectra. Mixed ligand complexes were also prepared by reaction of the chloro salt with ammonia, sodium nitrite, and sodium cyanide in various combinations in water. However, none of the UV spectra of any of these compounds matched that of the Rh leach solution. The hexacyano complex was still the closest match, despite its ill defined spectrum.
Ramifications of PGM Species Geometry: The identification of the Pt and Pd species as square planar cyanide complexes, and the Rh as an octahedral cyanide complex, helps explain some of the experimental results observed in earlier phases of this work. For example, it was found that recovery of the PGM from solution was difficult. Activated carbons were found to adsorb both Pt and Pd to some extent, but not to high enough loadings to effect a significant concentration factor after elution. In addition, it was found that Rh was not extracted by the activated carbon. This can now be explained on the basis of the speciation of the PGM complexes. Activated carbon is routinely used for gold adsorption, where the gold is in the form of the dicyanoaurate(I) ion, [Au(CN)2]. This ion is linear with a single negative charge. Research has shown that although neutral and singly charged anions are easily adsorbed by activated carbon, doubly and triply charged anions are adsorbed much less readily (Ibrado and Fuerstenau, 1989). For example, adsorption of copper onto activated carbon can be minimized by the addition of cyanide, which converts the predominant copper cyanide complex from [Cu(CN)2] to [Cu(CN)3]² or [Cu(CN)4]³. This results in an increase in anionic charge, which inhibits adsorption significantly. Similar effects must therefore be occurring with the doubly charged Pt and Pd complexes, and especially with the triply charged Rh complex. This explanation is probably applicable also to the solvent extraction and ion exchange results observed earlier in this project, where PGM extractions, especially Rh, were not complete.
An alternative approach to PGM recovery that was found to give complete recovery of PGM from solution was thermal decomposition. In this process, the solution was heated to 275° C in an autoclave for several hours. This was found to give complete removal of PGM from solution, with the added benefit that the total cyanide concentration was decreased to less than 0.2 ppm.
Leaching Rate Studies
The rate of dissolution of the PGM was the next factor to be investigated. From catalyst leaching tests, it appeared that Pt and Pd were more easily leached than Rh. However, more quantitative data in this area would be desirable. Also, the rates of dissolution processes are often controlled by the rate of a single step, or “bottleneck,” of the process. For a material that does not form a porous reaction layer, the steps are mass transfer of the reactants to the surface, chemical reaction at the metal surface, and mass transfer of the products away from the surface. If the rate limiting step of the dissolution process can be identified, it may be possible to increase the dissolution rate by increasing the rate of the limiting step.
PGM Foil Samples: Initially, rate studies were conducted using metal foil samples of Pt, Pd, and Rh. These studies were run under the usual conditions used for treatment of catalysts, with 0.1M NaOH, 1 pct NaCN in solution, at 160° C for 1 h. There was no agitation in these tests, as it would be difficult to reproduce the agitation level from test to test because of factors such as the orientation of the foil sample. The surface areas of the foils were taken as their geometrical areas. The exposed surface areas of these samples was of course much less than catalyst samples, but for Pd and Pt, enough metal was dissolved so that a weight loss could be detected, and as a more accurate measure, the solution could be analyzed for PGM content. Due to hydrolysis of cyanide, and to leaching during the heatup period, absolute leaching rates could not be calculated from these data. However, this procedure gives a reasonable duplication of the leaching procedure used for catalysts and can at least show differences between the PGM. Based on the area of the foil samples and the solution analysis, Pd leached 20 times faster than Pt, on a molar basis. For Rh, no measurable weight loss occurred and no Rh was detected in solution after the leach test. Therefore, only an upper limit can be assigned to the leaching rate of Rh based on the detection limit of Rh in solution. This limiting rate was less than one fifth of the Pt leaching rate, and the actual rate was probably less than that. As a general result, leaching at 160° C with no agitation dissolved Pd at a rate 20 times faster than the Pt, which in turn was at least five times faster than Rh.
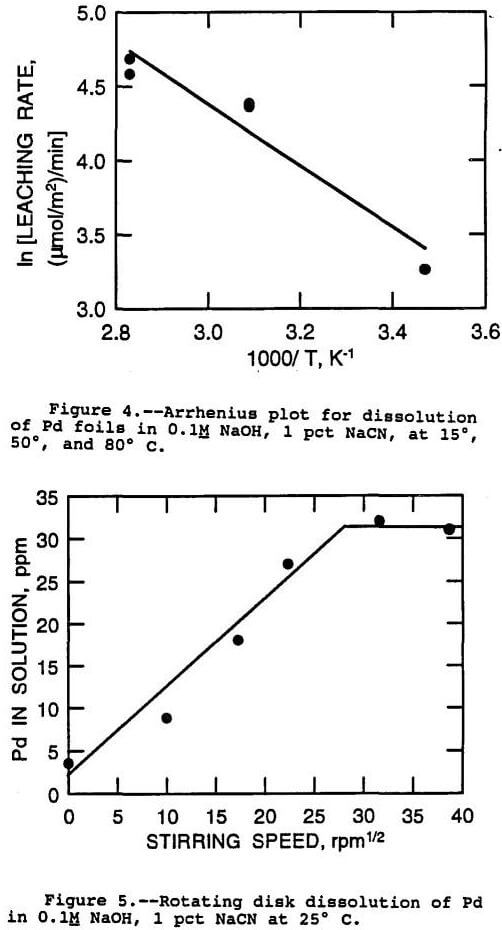
Additional rate tests were performed at lower temperatures with Pd foils. This was possible with Pd due to its relatively rapid rate of dissolution. Greater sensitivity was obtained by increasing the contact time of the test, up to 24 h at ambient temperatures. A leaching rate of 26 (µmol/m²) /min was found for Pd at 15° C. Leaching rates were also measured at 50° C, and at 80° C, and were found to be 79 (µmol/m²) /min and 103 (µmol/m²)/min, respectively.
The temperature dependance of a dissolution process can often be evaluated to find the rate determining step of the process. This is because chemical reaction rates generally show much higher sensitivity to temperature than diffusion rates. Therefore, if the rate of dissolution shows a high activation energy or a high sensitivity to temperature, then the process is probably controlled by the rate of the chemical reaction at the surface of the metal. On the other hand, if the activation energy is low, then the rate limiting step of the process is probably diffusion of reactants or products to or from the surface of the metal. Wadsworth has set out some numerical values for these rate controlling regimes, and gives less than 5 kcal/mol activation energies for diffusion controlled processes, and 10 to 25 kcal/mol for chemical reaction controlled processes (Wadsworth, 1979).
The procedure for determination of the activation energy is to plot the natural log of the reaction rate as a function of the reciprocal of the absolute temperature. This kind of plot is termed an Arrhenius plot. The data for dissolution of Pd from foils at 15° to 80° C was analyzed in this way, and the Arrhenius plot is shown in figure 4. An activation energy of 4 kcal/mol was obtained from the plot. This indicated a diffusion controlled mechanism according to the guidelines presented above.
Rotating Disk Experiments: Further tests regarding the mechanism of PGM dissolution were run using rotating disk samples. This technique was applicable only with Pd and Pt, as the dissolution rate of Rh was too low to be measurable with this technique. As shown by Levich (1962), the flux of material to a rotating disk is proportional to the square root of the disk rotation speed ω.
Information regarding the mechanism of dissolution can be extracted from the experimentally observed relationship between the disk rotation speed and the rate of dissolution. If the rate of reaction is controlled by transport of reactants to the disk, then the reaction rate will be proportional to the square root of the rotational speed of the disk. However, if the rate is controlled by the rate of chemical
reaction at the metal surface (and the flux of reactant is greater than this rate), then the rate will be independent of the disk rotational speed. The data is commonly plotted as the dissolution rate versus the square root of the rotational speed ω. What is often observed is a linear relationship, which levels out above a certain speed, where the mechanism changes from mass transfer to chemical reaction control.
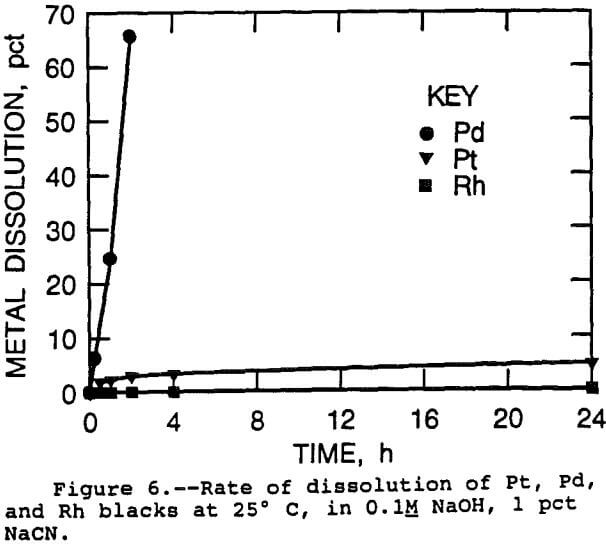
This was observed for a Pd disk in a solution of 1 pct NaCN and 0.1M NaOH at 25° C, as shown in figure 5. The critical rotational speed in this case was 950 rpm, with mass transfer control below that speed, and chemical reaction control above that speed. The reaction rate where the mechanism changed over was 700 (µmol/m²)/min. This is therefore the maximum leaching rate for Pd at 25° C in this solution. The leaching rate of the Pd disk where no stirring took place was 31 (µmol/m²)/min, less than one tenth of the chemical reaction rate. This agrees well with the rate obtained by leaching of Pd foil at 15° C, 26 (µmol/m²)/min. Note that the maximum reaction rate under well stirred conditions at 25° C was much faster than that measured for Pd foil under unstirred conditions at the much higher temperature of 160° C.
The rotating disk experiment was repeated with a Pt disk. However, even after 24 h at stirring speeds up to 1,000 rpm, less than 1 mg/L Pt was detected in 25 mL of solution. The Pt dissolution rate could be increased by heating; however, it was not possible to use the rotating disk system at elevated temperatures due to the difference in thermal expansion between the metal and the inert shroud. Still, the fact that the Pt concentrations were much less than those observed for Pd indicates that Pt dissolution must be controlled by chemical reaction, not mass transfer, since the mass transfer conditions were identical in the two test series.
PGM Black Samples: Powdered PGM black samples had much higher surface areas than foil samples; therefore, the amount of PGM solubilized per unit time was much higher. Thus, the use of PGM black samples gave the greater sensitivity that was needed to measure the dissolution rates of the Pt and Rh. For this reason, the investigation turned to the use of PGM blacks. Sampling from a pressurized autoclave was difficult, so these tests were performed under atmospheric pressure, at 25°, 50°, and 80° C. The leach slurries were well stirred, with the PGM blacks in complete suspension. Solution samples were taken at selected time intervals during these tests, giving the extent of dissolution as a function of time. The rate of dissolution of Pt, Pd, and Rh at 25° C is shown in figure 6. It is immediately apparent that the same order has been followed as with the foil samples, with Pd being solubilized most rapidly, followed by Pt and Rh. Leaching rates were calculated based on solution assays taken early in the test, at less than 10 pct extraction, to minimize the effect of the decrease in surface area caused by leaching. However, the calculated Pd leaching rate did not agree with the rates obtained from the Pd foil and rotating disk studies, especially considering that the blacks were leached in an agitated system. The variable most subject to error in PGM black leaching tests was the surface area of the blacks, upon which calculation of the leaching rate depended. The surface areas of the blacks were measured by the BET method using nitrogen adsorption, which is an accepted technique, but the determination was carried out under dry conditions. It may be that the particles aggregated when added to solution, especially at the high ionic strength and elevated
temperatures of some of these tests. In fact, some aggregation was observed during the course of the experiments, especially at elevated temperatures. This would lead to a decrease in available surface area, and rates calculated based on the BET surface area would therefore be less than the actual rate.
Application to Catalyst Leaching: From the experimental results discussed above, it appeared that diffusion was the rate limiting step for elemental Pd samples, but that chemical reaction was limiting for Pt and Rh. However, the situation was more complicated for catalyst leaching, since the catalysts were not pure samples of PGM. Therefore, catalyst leaching behavior may be different. For example, it was found that, in the autoclave leaching of Pt, Pd, and Rh black samples, no further extraction took place after the autoclave reached operating temperature. This was in contrast to the catalyst samples, where significant leaching of the PGM contained in the catalyst took place during the time at temperature. The main difference between the elemental PGM samples and the catalyst samples was that the PGM in the catalysts were widely dispersed on a porous matrix, but the elemental PGM were immediately accessible in a pure state. Thus, the rate of pore diffusion, which was a possible rate-limiting step for the catalysts, was not a factor for the elemental samples.
In addition, because of the fine size and small amount used in leaching, the blacks were usually leached under agitated conditions. However, agitation leaching of catalysts was not found to be beneficial, probably since the PGM are located within the pore structure, and thus not immediately accessible to the solution. Possible rate-limiting steps for the dissolution of PGM from catalysts were therefore either the rate of pore diffusion, or the rate of the chemical reaction at the surface of the metal.
The data from catalyst leaching tests published earlier were analyzed for the dependence of the leaching rate on the temperature. The resulting activation energies ranged from 1 to 6 kcal/mol, suggesting diffusion control. These leaching tests were conducted at temperatures of 25° to 80° C. However, since diffusion rates are generally less temperature sensitive than chemical reaction rates, diffusion is likely to still be rate controlling at 160° C. Additional support for the pore diffusion mechanism can be found in the results from a leaching rate test conducted with the Johnson-Matthey catalyst. In this case, Pt and Rh were still appearing in solution long after the cyanide had been depleted. Based on the relative sizes and shapes of the PGM complexes, Pt and Pd should be leached at roughly the same rate, while Rh would be slower, due to the greater size of the octahedral complex. This is also consistent with catalyst leaching data.
The rate of pore diffusion cannot be increased by mechanical means, that is by stirring, but can be increased by an increase in temperature. However, the cyanide complexes, especially Pd, begin to precipitate above 160° C. Therefore, it appears that it will not be possible to increase the dissolution rate for the catalyst samples above that observed at 160° C.
Conclusions
Unlike ambient temperature cyanide leaching processes, those at elevated temperatures show rapid loss of cyanide due to hydrolysis to ammonia and formate. The half life of cyanide at 160° C is about 7 min, therefore, there is little point in leaching for extended periods of time at elevated temperatures.
For Pt and Pd dissolution in cyanide solutions, the relevant cyanide complexes were identified as square planar tetracyanoplatinate(II) and tetracyanopalladate(II) species. This was proven by their distinctive UV spectra. For Rh, the geometry of the cyanide complex was found to be octahedral, as the hexacyanorhodate(III), rather than the square planar geometry seen for Pt and Pd. Although the UV spectra for the Rh cyanide complexes were much less distinctive, the match was evident. This difference in geometry and charge density is the most probable explanation for the behavior of the PGM in various solution treatment steps such as carbon adsorption, solvent extraction and ion exchange, where Rh extraction was observed to be much less than extraction of Pt or Pd.
The rate of dissolution of elemental. PGM samples was measured by three different techniques. These were metal foil leaching, metal powder leaching, and rotating disk leaching. For the metal foil samples at 160° C, the rate of solubilization was greatest for Pd, followed by Pt and Rh. The ratios of the leaching rates were 20 to 1.0 to less than 0.2. for the Pd, Pt, and Rh, respectively. From the temperature dependence of Pd dissolution from foil samples, it was determined that the rate limiting step was due to mass transfer considerations. This was confirmed by rotating disk tests using a Pd disk. Since the leaching rate from a Pt disk was much less than that of Pd under identical conditions, the Pt dissolution process must be controlled by the rate of the chemical reaction at the metal surface. Leaching tests using PGM blacks confirmed that the rate of solubilization increased in the order Rh, Pt, and Pd, but due to uncertainties in the surface areas of the samples, absolute leaching rates could not be calculated.
The rate of pore diffusion appears to be the limiting step for PGM dissolution from catalyst samples. Diffusion rates can be increased by an increase in temperature, but the stability of the Pd cyanide complex sets the upper temperature limit at 160° C.
