In October of 1987, the U.S. Bureau of Mines (Bureau) initiated a study on the applicability of cyanide technology to the Alaska mineral industry. The study included the collection and analysis of cyanide literature and leaching costs, as well as the generation of several generic feasibility studies. Although other methods of cyanide use in the mining industry such as in situ and vat leaching have been examined, heap leaching is the main focus of the study.
This report discusses heap leaching methodology and evaluates the capital and operating costs of a “typical” Nevada heap leach operation of variable tonnage as it would exist in Interior Alaska. Cost data was generated by the Bureau’s Cost Estimation System (CES), Western Mine Engineering’s Mining Cost Service (MCS), and published case histories. The objective of these analyses is to compare the hypothetical costs of heap leach mining in Alaska to those of Nevada.
Heap leaching is a metal recovery method which typically uses a sodium cyanide solution to dissolve and remove precious metals from low-grade ore that is stacked into piles. In the last decade, the number of mines using this technology has mushroomed with the majority of this increase occurring in the Western United States. In 1986, the U.S. heap leach production was 31,060,048 g (998,603 oz) of gold and 113,270,891 g (3,641,741 oz) of silver. This amounts to a 20-fold increase in leached gold and a 125-fold increase in leached silver over 1.979 production figures.
Such growth is especially surprising when the low gold prices of the mid- and late-1980’s are considered. The popularity of heap leaching can be attributed to the low capital and operating costs associated with this mining method. Deposits considered uneconomic to mine with conventional methods can be heap leached at a profit. With the use of agglomeration techniques developed in the early 1980’s, as well as drip emitters and solution heating, mines with clay-rich ore or located in colder climates, or both, are now able to heap leach successfully. When Pamour, Inc. (now a division of Giant Yellowknife Mines) began a successful heap leach operation in Northern Ontario in 1987, Alaska’s many low-grade deposits appeared more attractive as development targets.
La Teko Resource’s Ryan Lode, near Fairbanks, became the first heap leach operation in Alaska in 1987, successfully leaching a mineralized quartz-schist shear zone averaging 4.5 g/mt (0.13 oz/st) gold. Fairbanks Gold Mining Company is currently exploring the Fort Knox deposit near Fairbanks as a possible heap leach operation. On average, Fort Knox ore has just 1.37 g/mt (0.04 oz/st) gold.
Acknowledgments
The author wishes to thank the personnel of the Western Field Operations Center, U.S. Bureau of Mines, Spokane, Washington for their assistance in obtaining cost data and information pertaining to environmental regulations. The author also thanks Roger Burggraf and Ed Armstrong of Tri-con Mining, Inc., and Chris Babcock of Citigold Alaska, Inc. for tours of their facilities and for sharing their knowledge of processing with cyanide.
History of Cyanide Use
The principle of cyanidation is attributed to Doctors Robert and William Forrest and John MacArthur, a self taught chemist. After years of research in their homemade laboratory in Scotland, they were issued a British patent in 1887. Subsequent patents were given in the U.S. in 1889. The process involved the use of potassium cyanide (KCN). The finely ground ore was agitated in the presence of air and cyanide solution before precipitation with zinc. Cyanidation revolutionized gold recovery systems which had previously relied on gravity separation.
Dissolved gold was recovered exclusively by zinc precipitation until the early 1950’s, when surplus activated carbon used in World War II gas masks was available at bargain prices. In 1952, the Bureau first published a method of gold and silver recovery from loaded charcoal. With this process, a boiling NaOH-NaCN solution was passed through the loaded carbon, and then sent to an electrolytic cell for gold/silver deposition onto steel wool. With the advent of this method, carbon absorption became more economic than zinc precipitation.
Heap leaching was first documented in the mid-sixteenth century, when Hungarian mines recycled copper-bearing solutions through waste heaps. Spanish miners applied acid solutions to oxide ore heaps around 1752. Heap leaching for gold, however, was not commercially implemented until the late 1960’s. The first company to use this method was Carlin Gold Company in northern Nevada. Cortez Gold Mines began the first large-scale heap leach operation when it leached two million tons of ore in the early 1970’s. During the mid- 1970’s, the Bureau introduced the agglomeration technique which allowed heap leaching of deposits with clay and low permeability. With agglomeration and improvements in solution application, heap leach operations are possible with many climatic conditions and ore types.
Cyanide Chemistry
Cyanide’s success with gold and silver extraction is a function of its ability to associate with metals and carbons. When a sodium cyanide solution is exposed to oxygen, it solubilizes the metals into complexed metallo-cyanides. Research indicates that dissolution is bimodal. Most of the precious metals are dissolved by the following reaction (where X refers to the precious metal component):
2X + 4CN- + O2 + 2H2 → 2X(CN)2- + H2O2 + 2OH-
The remaining precious metals are dissolved in the traditional Elsner’s Equation (9):
4X + 8CN- + O2 + 2H2O → 4X(CN)-2 + 2OH-
Successful leaching of gold or silver ore depends upon the amount of cyanicides contained in the ore. Cyanicides are those elements or compounds that react with solutions to inhibit metal dissolution by combining with the cyanide, thereby causing excessive reagent consumption. These cyanicides include copper, sulfides, arsenic, and carbons. Ideally, the gold or silver should occur as free, fine, clean particles for efficient reaction. In decreasing order, relative- solubility of metals in cyanide solutions occurs as follows (10):
Mg+, Al, Zn, Cu, Au, Ag, Hg, Pb, Pt+
Elements located to the left of gold in the above list will consume the cyanide before it can combine with gold or silver. The presence of these preferential metals in the ore will result in lower precious metal recovery and excessive cyanide consumption.
Refractory ores, such as those rich in sulfides, are enormous consumers of cyanide. Sulfide ores are leachable, but only if the gold is free and not entrained in sulfide minerals. Ores in which the gold is not free may be treated in one of several ways. Unfortunately, each method is costly. The simplest method is to expose the ore to the environment and allow the sulfides to oxidize. This process may take many years, however, to produce a leachable ore. In some cases, gold has been successfully leached from old mine tailings where initial recovery was poor due to the presence of sulfides. In such cases, the sulfides have had fifty or more years to oxidize. A second method, based on bioleaching, has proven effective at Giant Bay, Canada. In this process, bacteria, such as thiobaccilus ferrooxidans, is added to the ore. The bacteria metabolizes the iron, resulting in the breakdown of the iron-bearing sulfide and the release of the entrained or encapsulated gold. Other methods also used include chlorination, pressure oxidation with autoclaves, and fluid-bed roasters.
Cyanide Heap Leaching
The attractiveness of heap leaching is its low cost. In a heap leach operation, ore is usually surface-mined. For flat-lying, near surface ore bodies, this method is considerably less expensive than underground mining methods. Higher grade ore is generally vat leached instead of heap leached. This is due to the fact that heap leach operations have traditionally lower recovery values than those of vat leaching. Also, in higher grade ores, gold grains are often larger, and thus have a larger surface area. If this ore is heap leached, the gold dissolution time is longer than with an agitated vat cyanide solution, where ore is usually more finely crushed and more thoroughly wetted with solution.
Once the ore is mined, the ore is hauled to the leach site for pad emplacement by conveyor or radial stackers, loaders, or dump trucks. The heap leach pad typically consists of a lower liner composed of 30 cm (12 inches) of compacted soil/clay with permeability of 10 -6 cm/second or less. This layer is covered with a 30 cm (12 inch) thick leak detection layer of highly permeable sand or rounded, small diameter gravel. This layer is covered by a 20-80 mil synthetic primary liner. Finally a 60 cm (24 inch) layer of permeable material such as crushed and screened ore, is placed on the upper liner to protect it from tearing during ore emplacement, Perforated polyvinyl chloride (PVC) piping with holes drilled at 5 cm (2 inch) spacing is installed in this layer for solution collection. Notwithstanding environmental compliance, it is important to construct an impermeable heap pad liner system on a solid foundation. If the pregnant (containing recoverable quantities of precious metals) solution is allowed to escape through a faulty liner system, the economic viability of the operation is diminished.
The objective of heap construction is to build a stable, yet porous, heap which allows even distribution and percolation of heap solutions throughout the heap. Even distribution is necessary for the leach solution to reach as much of the precious metals in the ore as possible. After ore emplacement on the leach pads, a barren (not containing recoverable quantities of precious metals) cyanide solution is added via sprinkler or drip-line to the heap at an average rate of 0.002 to 0.003 l/s/m² (0.003 to 0.005 gpm/ft²). The ore needs to be permeable enough for the solution to percolate through the heap and dissolve the gold. For ores that have been found to have low metal recovery using conventional leaching methods, recovery can be enhanced by crushing and/or agglomerating the ore. Crushing the ore creates larger surface areas on ore particles, thereby increasing the probability that gold particles will be directly exposed to cyanide solutions. Crushing ore to a small grain size can, however, inadvertently cause other problems. Fine grained and clayey materials reduce heap permeability, and can cause cyanide solutions to pond or channel. To solve this problem, the Bureau in the mid-1970’s developed a process known as agglomeration. Agglomeration is the process where cement and moisture are added to the ore. The mixture is tumbled before or during placement on the pad. This results in the formation of balls of cemented fines which enable cyanide solutions to permeate the heap, while preventing the solution from ponding on top of the piles or channeling in the heaps. The average amount of cement added for agglomeration is 5 kg/mt (10 lb/st) ore. Tests have shown that the optimal size of agglomerates is 1.9 cm (¾ inch) with a moisture content of 12%. Instead of water, a cyanide solution is often added to increase moisture content. This gives the leaching process a head start over heap solution application alone. Agglomerates are often placed on the pad by conveyor. The ore/cement mixture forms into agglomerates by tumbling off the end of the conveyor onto the pad. This system also minimizes heap compaction and maximizes heap permeability relative to other pad emplacement methods such as loader or truck dumping.
Once the cyanide solution passes through the heap, it is collected in lined ponds and pumped through carbon columns. The columns are plastic or fiberglass containers filled with crushed carbon produced from charred peach pits or coconut hulls. Coconut carbon is generally preferred because of its lower cost and higher porosity, hence surface area. The diameter of, and height of the carbon columns are determined by pilot testing in the development stage. Typically, the pregnant solution passes through a series of these columns. The gold and silver in the pregnant solution adsorb onto the carbon. The now barren solution is recharged with cyanide and the pH is adjusted before being reapplied to the heaps. Once a carbon column is loaded with anywhere from 6,856 to 24,400 g of gold per metric ton (200 to 800 oz/st) of carbon, the column is stripped using a hot caustic solution which is electrolyzed on steel-wool. The steel wool is then smelted, leaving a gold and silver dore.
Another method of gold extraction from pregnant cyanide solutions is the Merrill-Crowe, or zinc precipitation, process. As observed from the above list of cyanide-soluble metals, zinc has a higher affinity for cyanide than does gold. Zinc dust is added to clarified, de-aerated solution. During the zinc-cyanide reaction, gold and silver are precipitated, filtered, and sent to the smelting furnace.
The choice between these two methods in a gold recovery system depends on the ratio of silver to gold in the ore. If there is a concentration of greater than five parts silver to one part gold, the silver will load more quickly on the carbon columns, leaving the gold to load last, if at all. In this situation, zinc precipitation method is used. With a ratio of less than five parts silver to one part gold, the carbon-column method is generally preferred.
Environmental Factors
The 1989 U.S. production of cyanide was 72 million kg (160 million lbs). Half of the total production went to Nevada, which produced 65% of U.S. gold. In the nearly 100 years that cyanide has been used in the mining industry, there has never been a reported mining-related death from cyanide.
Cyanide’s ability to compound with metals accounts for the fact that it is a deadly poison. In the human body, cyanide inhaled or ingested at a lethal dose of 100 mg passes into the blood stream, where it bonds with iron. When the cyanide contaminated blood gets into the lungs, iron is no longer free to collect oxygen, hence the person suffocates. It is important to note that cyanide can also be absorbed through the skin.
Small quantities of cyanide can cause weakness and dizziness, but not death. Smaller cyanide doses are metabolized by the body and passed with wastes. Effects of chronic, low-level exposure to cyanide are non-specific and rare. Cyanide is also non-cumulative, non-carcinogenic, and non-embryotoxic. It should also be noted that this chemical occurs in nature, and is present in such foods as peaches, almonds, lima beans, and cigarettes. Cyanide as a fixed nitrogen has widespread use in agriculture as a fertilizer. In industry, cyanide is used for the case hardening of steel, for metal cleaning; it is used to produce drugs and vitamins, dyes and pigments, polymers, catalysts, and metal coatings of brass, cadmium, copper, gold, and silver, and zinc.
Cyanide is easily attenuated in the environment. Table 1 contains a synopsis of the natural detoxification modes of sodium cyanide. Cyanide can also be detoxified by application of hydrogen peroxide, chlorine, sulfur dioxide and air (the Inco process), and ferrous sulfate.
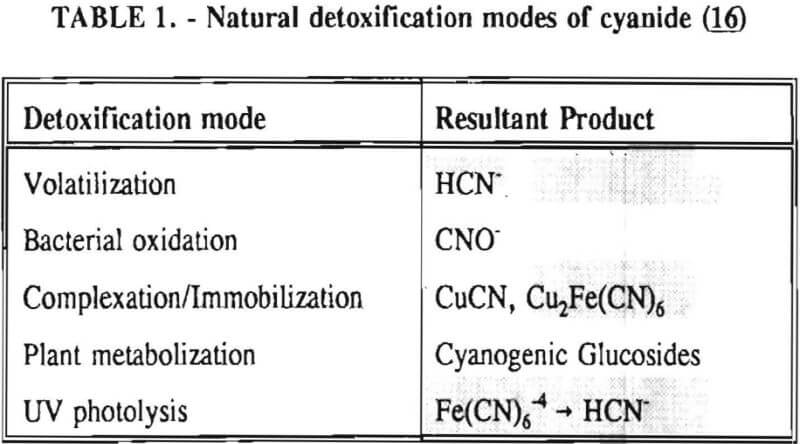
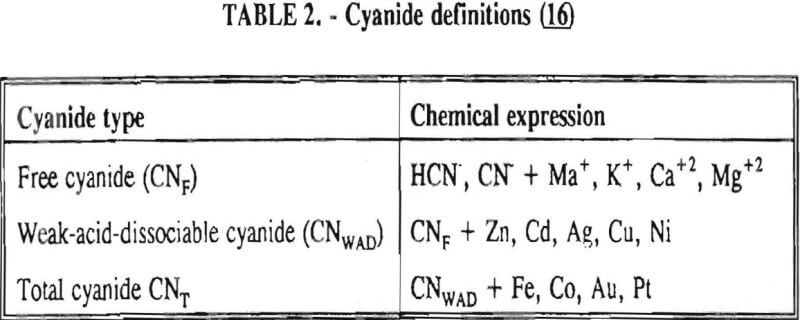
In the environmental community, there are several different types of cyanide referred to in regulations. These definitions are listed in Table 2. Weak-acid-dissociable (WAD) cyanide analysis will determine the amount of free (chemically reactive) cyanide present as well as the more stable, less toxic compounds listed in the table after CNWAD. Total cyanide analysis will reveal the amount of free cyanide, plus additional compounds detected with the WAD techniques, as well as the more stable compounds lists in the table after CNT. Free cyanide analyses are often unreliable due to many possible interferences from other compounds and elements.
Unfortunately total cyanide analyses are often confused with free cyanide analyses in environmental regulations, causing unrealistic standards.
Since heap leaching is new to Alaska, the problem of environmental permitting for cyanide projects exists. Most state regulatory agencies in Alaska are only now being educated in the use of cyanide. Nearly one hundred years of responsible use of cyanide in the mining industry, however, has proven that cyanide is safe and effective. Since the dissolved gold in the cyanide solution represents the miner’s profits, it is in his interest to control spills and other accidents.
The Federal Resource Conservation and Recovery Act (RCRA), Subtitle D (40 CFR Part 261, Subpart D, 1985) does not yet classify wastes generated in mineral production as hazardous waste, but as solid waste. Empty drums of sodium cyanide are considered hazardous wastes until the drums have been triple rinsed. Also, all spills and discarded products associated with sodium cyanide may be considered hazardous.
The Alaska Department of Environmental Conservation (DEC) began a regulatory campaign in 1988 requiring less than 0.02 ppm of free cyanide in waste water. Heaps must be equipped with double-lined pads and monitoring systems. Monitoring systems provide continuous cyanide leakage detection for groundwater and vadose zone contamination. Pond volume must be large enough to contain a 100 year, 24-hour storm event. Seasonal closure requires the neutralization of cyanide in process water which may be accessible to wildlife or may potentially cause freeze damage over the winter and leak out of the system. For mine closure requirements at the end of the mine life, waste water must be neutralized and the heap must be capped and recontoured. The cap must prevent percolation and infiltration of 90% of the average annual precipitation to the wastes and be able to withstand damaging freeze-thaw cycles. The operator is required by the DEC to maintain the cap and monitoring systems for a minimum of five years.
Alaskan Considerations
Heap leaching in Alaska was first successfully implemented by La Teko Resources, Ltd. after it acquired the Ryan Lode property east of Fairbanks in 1985. The deposit contains approximately 1.9 million tons of ore in a quartz-schist shear zone that averages 0.13 oz/st gold. After experimentation with test columns and heaps, the company began production in 1987. The operation places agglomerated ore on impermeable double-lined geomembrane pads and uses drip lines to apply a sodium cyanide solution. Pregnant solution is processed through a conventional carbon column circuit consisting of five carbon columns. Alaskan heap-leach operations face many problems unique to northern climates. Cold weather is the primary concern. Not only does the solution freeze, plugging sprinklers and lines, but the solution reaction itself noticeably slows from its normal dissolution rate at room temperature. The Ryan Lode operation avoids these problems by operating from mid-May to the beginning of October. The short leach period creates problems including a short period of cash-flow, in addition to yearly start-up and shut-down expenses. The leach period could be extended by heating the solution or the heaps, however, the expense, especially in severe temperatures, could exceed the revenue.
Another way to increase the leaching season in Alaska is with the use of buried drip emitters. Buried emitters have been used with great success in mountainous areas of Nevada. The Coeur-Rochester Mine in northern Nevada was the first to use buried drip emitters in order to operate year-round. The lines are buried 1 m (3 ft) beneath the surface of the heaps, decreasing the environmental effect of the weather and glaciering of the solution. Solutions can only be kept from freezing, however, if a constant flow is maintained. If the flow is disrupted for any reason, the line involved will freeze solid. By the use of drip, or pressure, emitters Alaskan mines should be able to increase their leach season to at least 175 days in Interior Alaska. It is doubtful, however, that buried drip emitters could withstand the rigors of an entire Interior winter without freeze-up or scaling problems.
Both capital and operating costs are higher in Alaska than in the contiguous U.S. The limited transportation infrastructure also adds to the cost of property development and limits the areal extent of economic heap leachable deposits. As transportation costs increase with distance from existing water or land routes, higher grade deposits must be exploited to maintain acceptable profitability. Additionally, the necessity for free gold in ore relatively free of cyanicides further reduces the number of leachable deposits.
Permafrost is another area of concern in Alaska. Pad emplacement on permafrost can result in thaw subsidence and frost jacking, leading to tearing of the liner system and solution leakage. This is not only an environmental concern, but also an economic concern. Solution loss means loss of dissolved gold and profit. Fortunately, most known deposits are located on thaw-stable ground. Pad placement on properties must consider permafrost. A site should only be used if it has good drainage, low moisture content, or both.
If an unstable permafrost site is used, several steps may be taken to minimize damage. Pads can be insulated with a gravel base similar to those used in roadways. Since this generates considerable capital expense, it should only be used in extreme circumstances. Once ore is placed on the pad, the ore itself will act as an insulator from the weather. An area of concern, however, is the edge of black polyethylene liner around the perimeter of the heap. The edge acts as a heat conductor to the ground, possibly resulting in subsidence and eventual collapse of the heap itself. A white cover or paint around the perimeter may reflect enough solar heat to minimize thawing. Ammonia freeze pipes such as those used on the Trans-Alaska pipeline are another possibility. These pipes are filled with ammonia, which vaporizes at warm temperature and condenses in cold weather. By circulating ammonia in the pipes, the ground is maintained in a stable, frozen state.
Another consideration is that solution heating to increase season length could cause warming of the heaps and underlying permafrost. When warming occurs, any insulating qualities the heaps provide for the permafrost are lost. Subsequent thawing of the liner base occurs along with settling and frost-jacking.
Cost Analyses
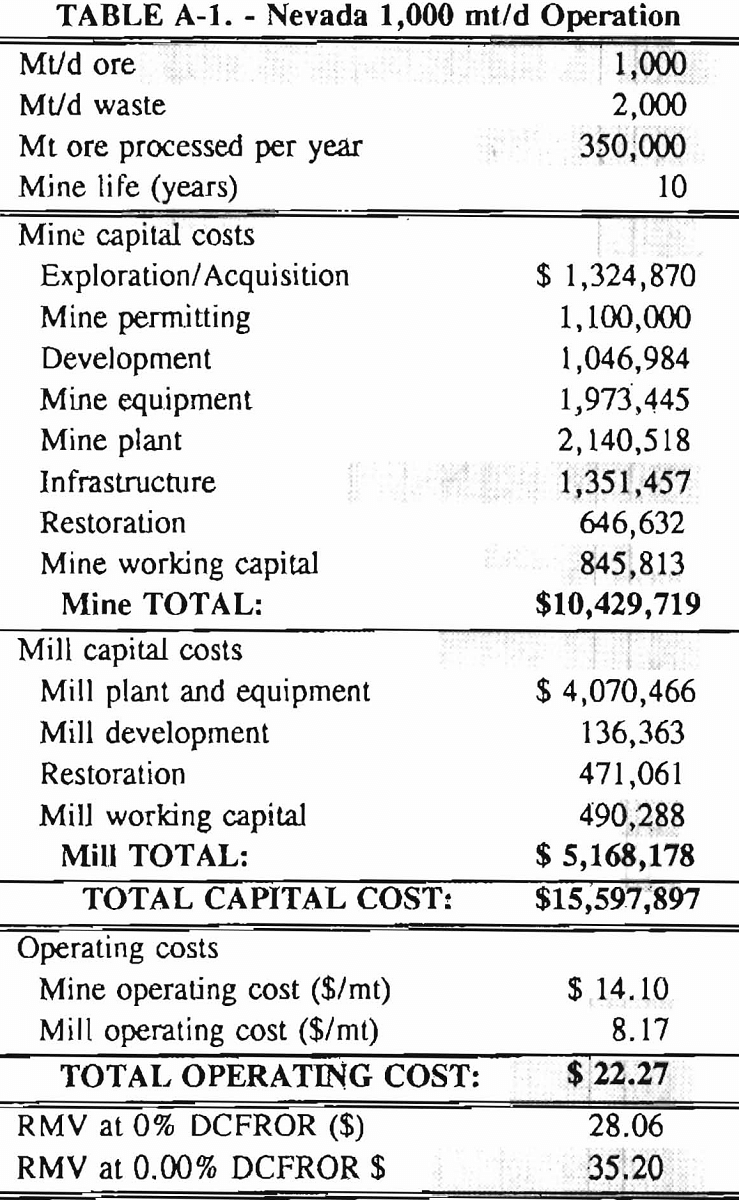
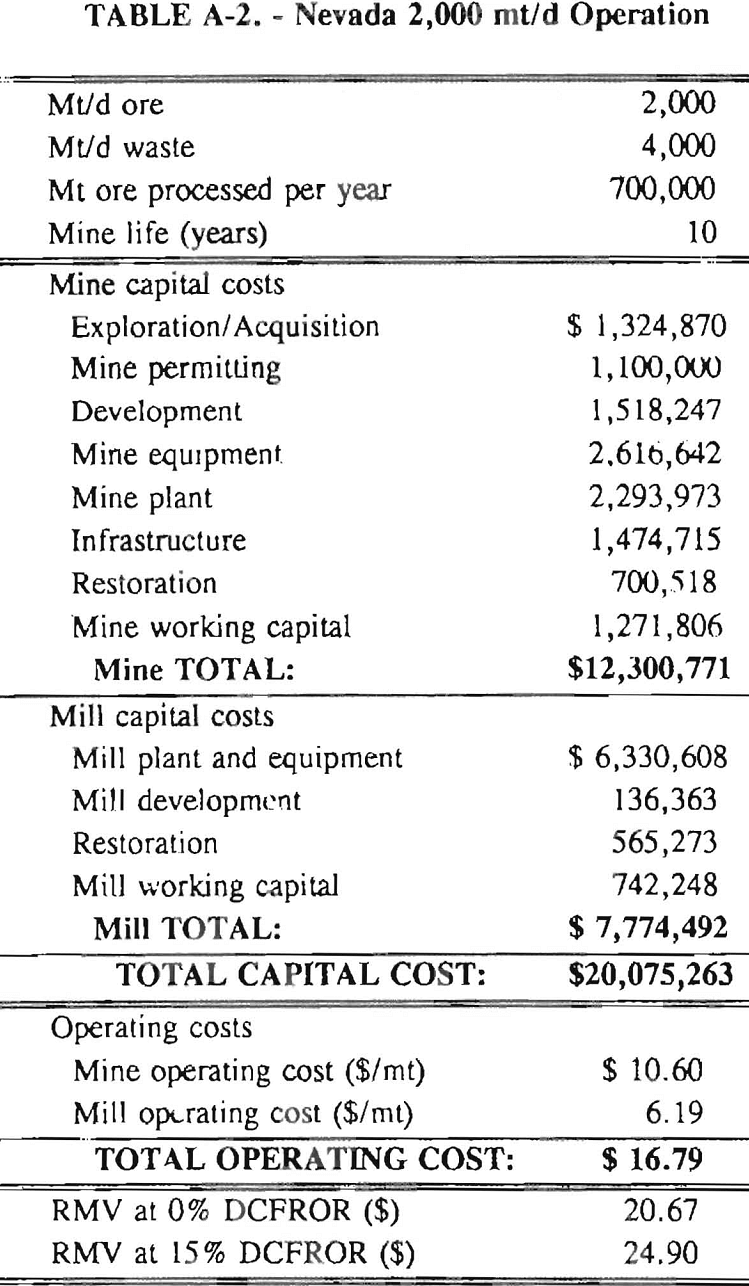
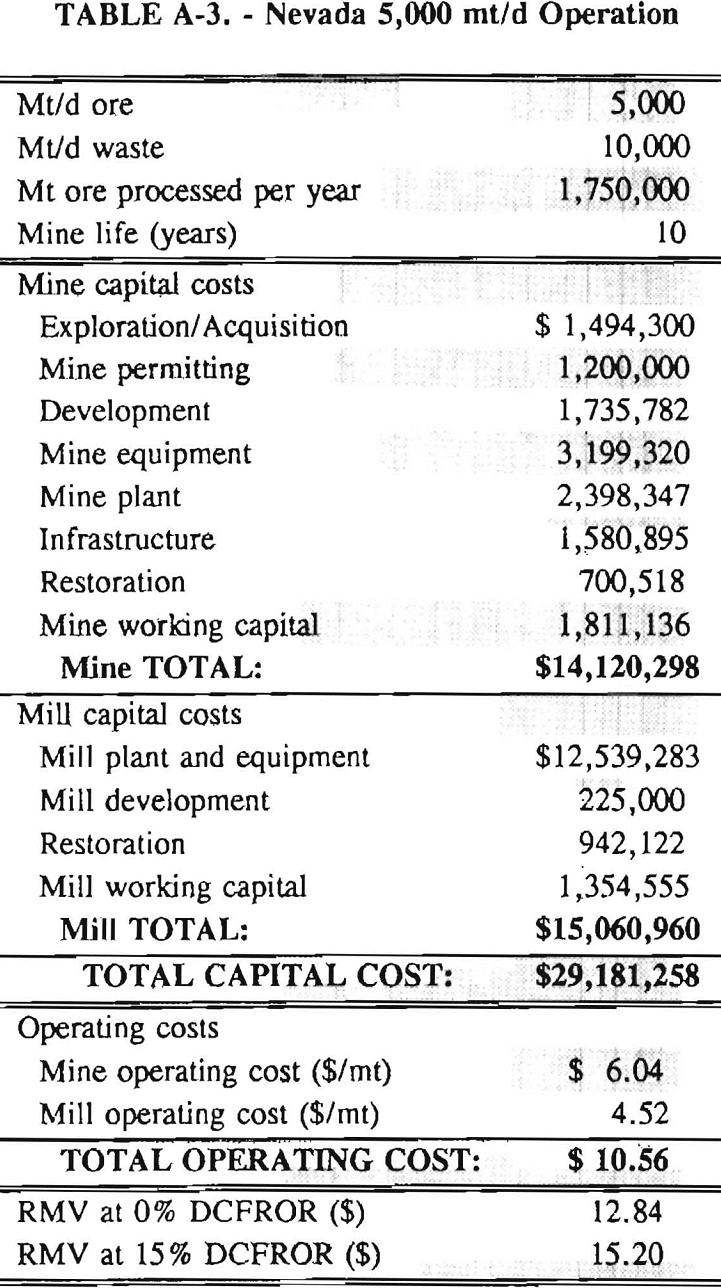
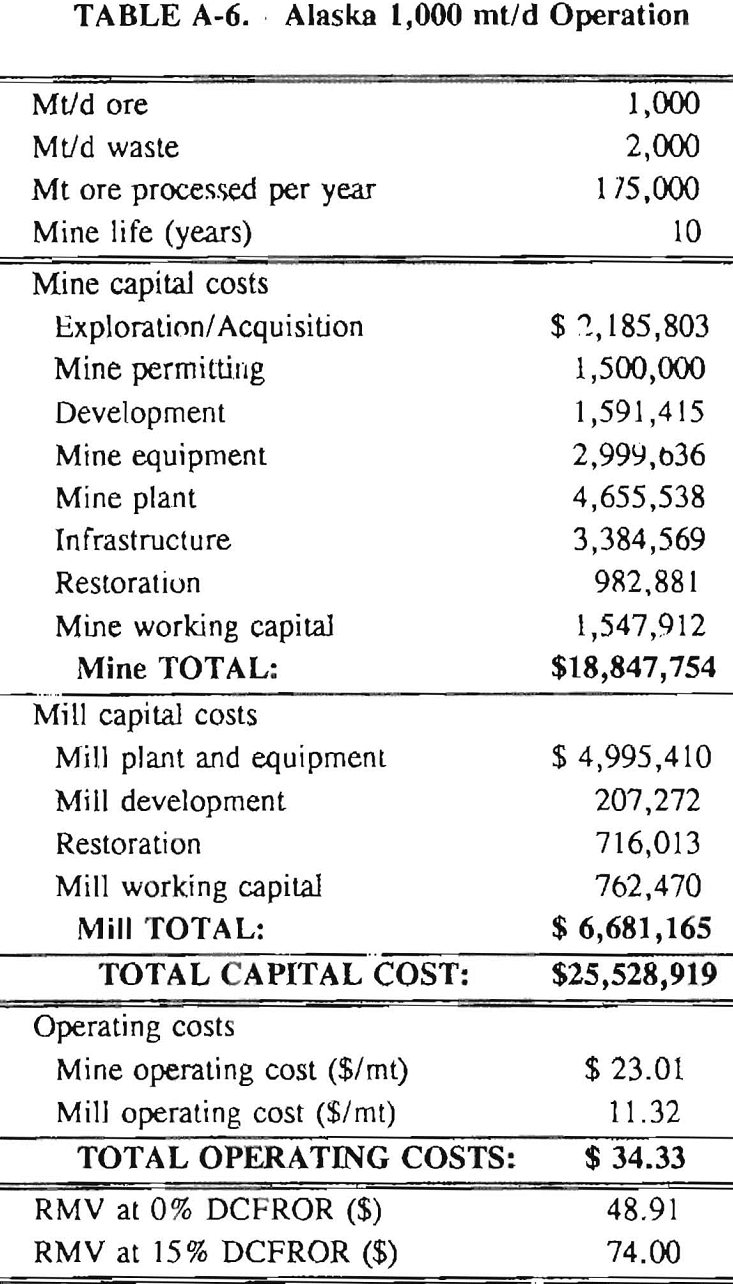
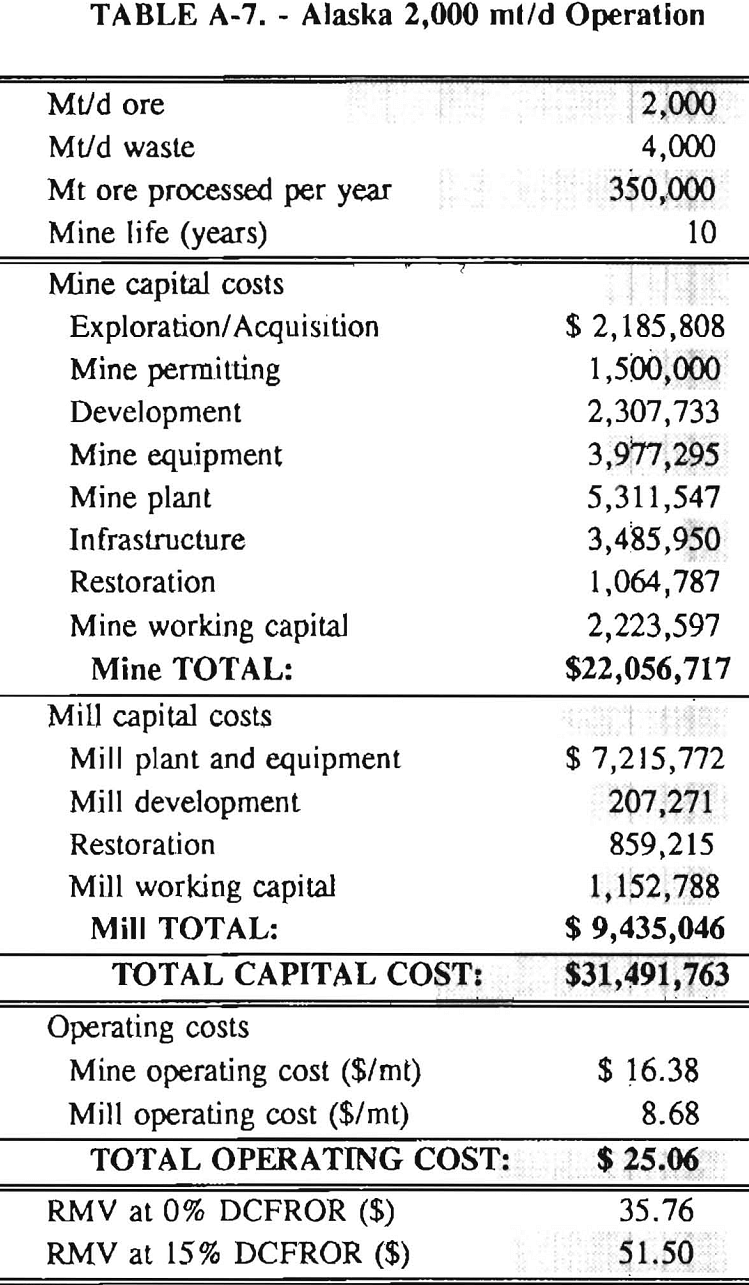
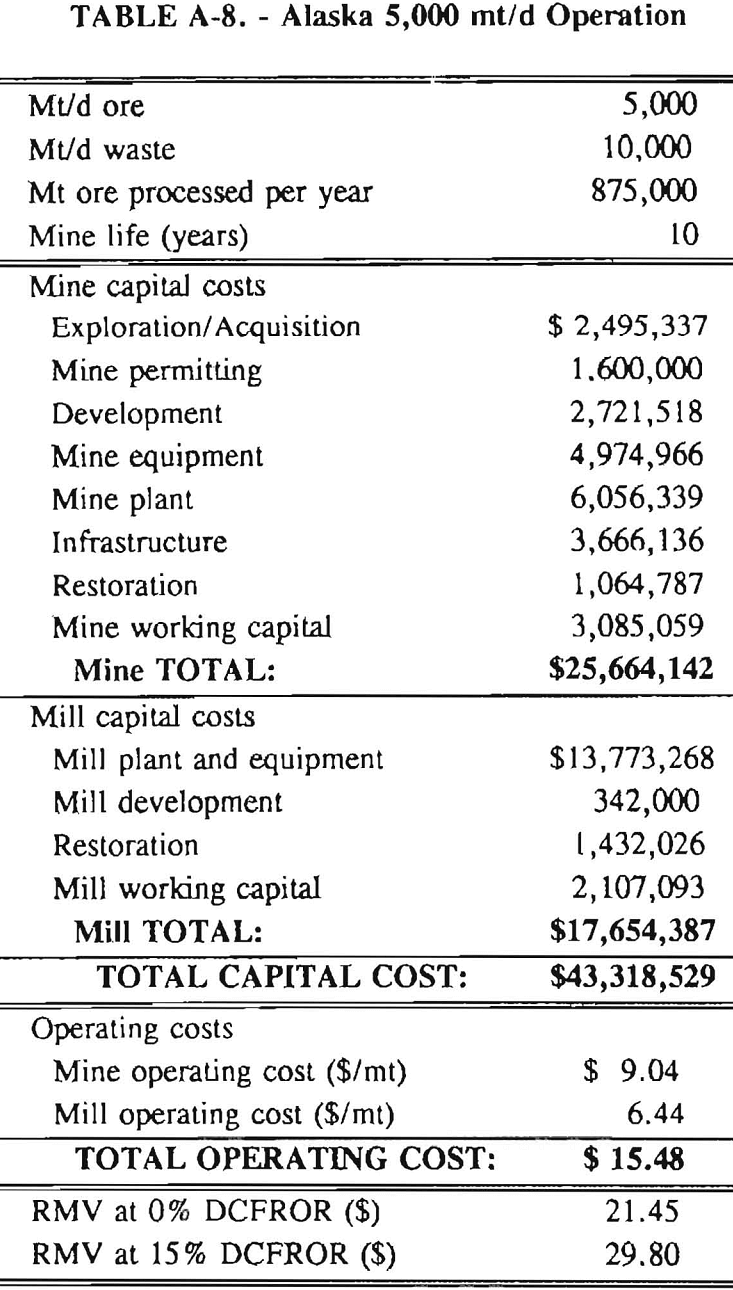
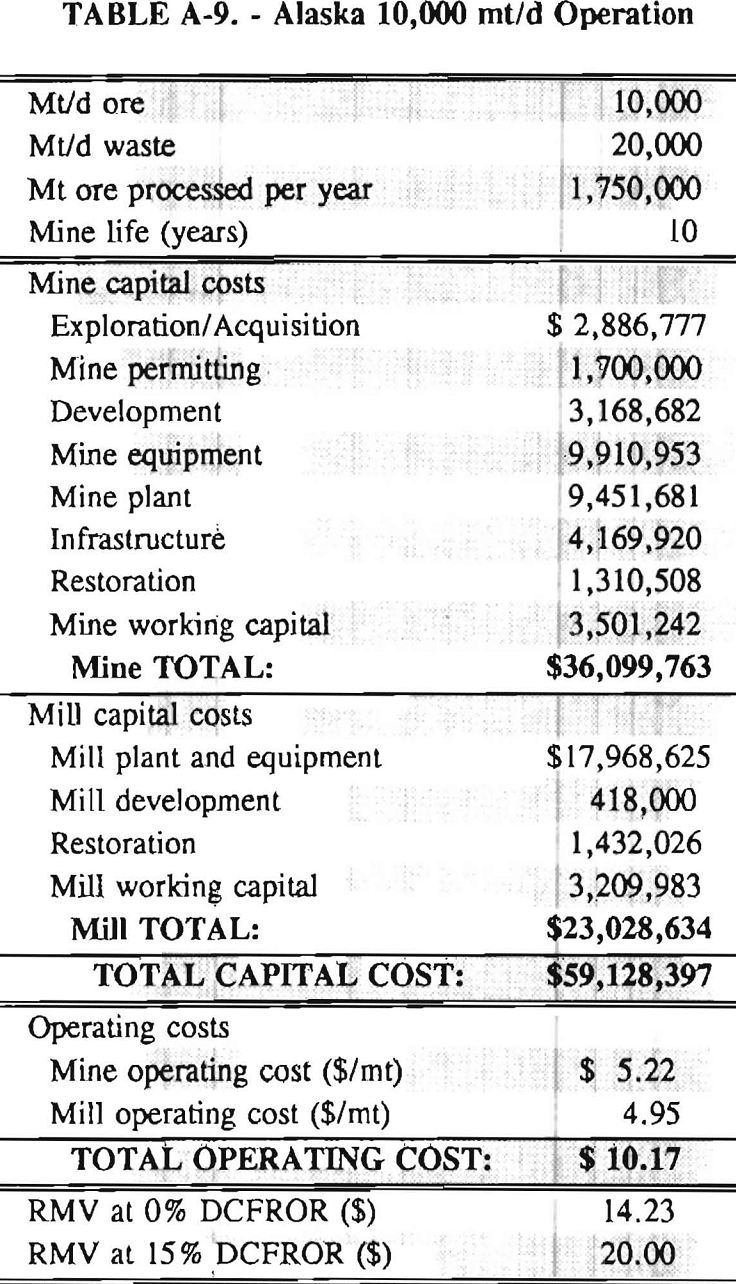
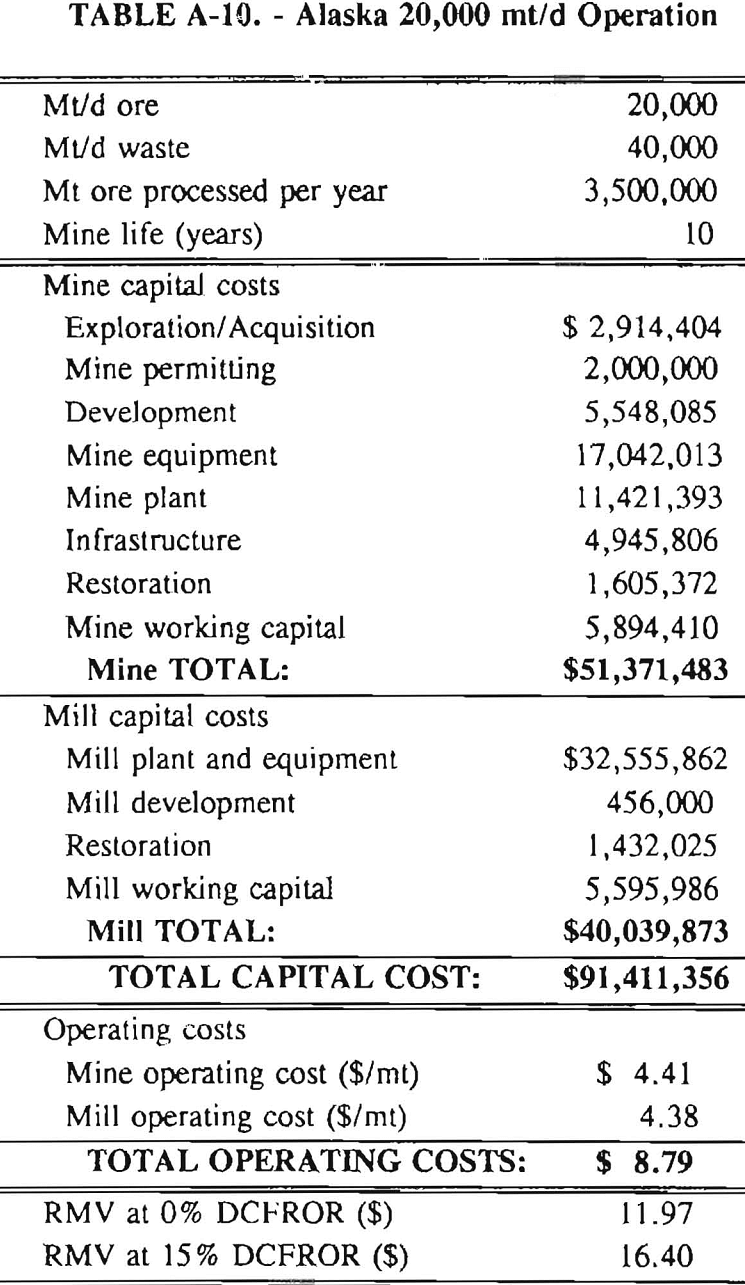
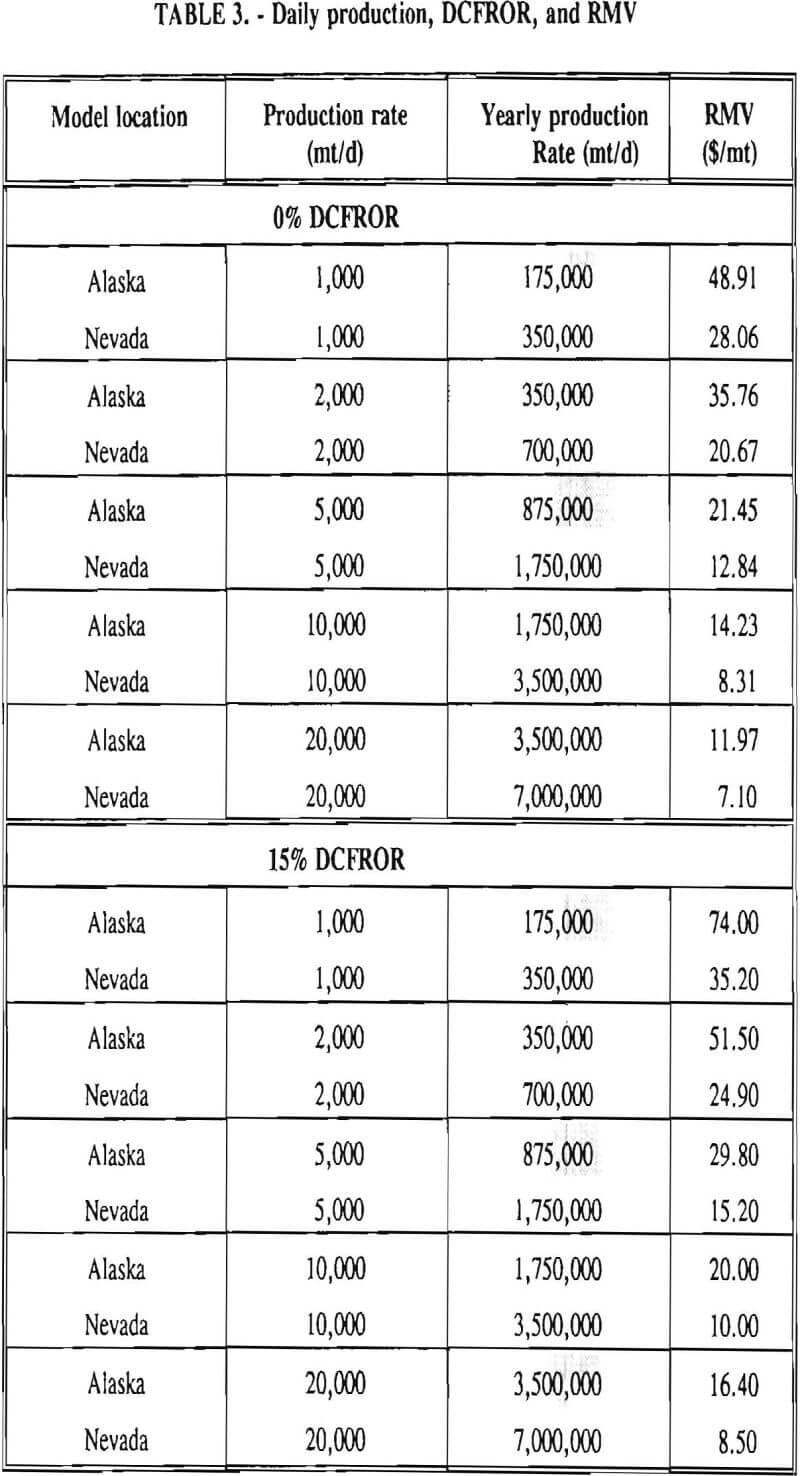
In 1987, the Bureau initiated a study on cyanide use and its applicability to Alaskan conditions. Concomitant to the collection of cyanide methodology, Bureau personnel obtained costs of heap leach mines in both Nevada and Alaska. Cost data and parameters were amalgamated in an effort to design a “typical” Nevada heap leach operation. Capital and operating costs were then calculated for this operation. The results were compared to the costs of an analogous hypothetical mine in Interior Alaska. Five daily tonnage rates were analyzed for both the Nevada and the Alaska operation (Table 3). The DCFROR for each production rate was then evaluated in terms of the RMV. The Nevada RMV for each production rate was then compared with its Alaska counterpart in an effort to relate the hypothetical costs of heap leach mining in Alaska to those of Nevada. RMV, as discussed later, was used to negate the fluctuations in metal market costs and recovery rates.
Mining and beneficiation costs were generated for two hypothetical heap leach models, one in Nevada and one in Alaska. In both cases, an effort was made to keep each model typical of its location. Each mine model was evaluated according to five different daily tonnage rates. Parameters such as crushing size, heap lift height, and access road length were kept constant. Both operating and capital costs were determined using the CES, the MCS, and published case histories. Costs summaries tor all mine and mill models are located in Appendix A. Alaskan operating costs were multiplied by 1.16 for labor and 1.52 for equipment and supplies to allow for the increased costs in Alaska compared to the western contiguous United States. An overall cost multiplier of 1.52 was also added to Alaskan capital costs to account for shipment of equipment to and within Alaska.
Nevada Mine Models
An open pit mining plan is assumed for the Nevada heap leach operation. The ore body is assumed to be tabular, flat, and near-surface, approximately 60 m (197 ft) in depth. The stripping ratio is 2:1 waste to ore. The mine model was economically evaluated at various production rates: 1000 mt/d (1,102 st/d); 2,000 mt/d (2,204 st/d); 5,000 mt/d (5,511 st/d); 10,000 mt/d (11,022 st/d); and 20,000 mt/d (22,046 st/d). Both ore and waste are drilled and blasted. Mines with production rates less than 10,000 mt/d ore (11,022 st/d) load ore and waste into trucks with front-end-loaders. Mines with production over 10,000 mt/d (11,022 st/d) load material into trucks with electric shovels. Waste is hauled an average distance of 750 m (2,460 ft) to dumps outside of the pit. Ore is hauled an average of 1,500 m (4,920 ft) to the heap leach pad emplacement area.
The electrical rate is assumed to be 0.05 $/kW·h and is provided by 10 km (6.2 mile)-long power lines. Line capacity increases with daily tonnage. Access roads are 6 m (20 ft) wide and 16 km (10 miles) in length. Neither camp nor town site costs have been included in the costs, since personnel will commute from the nearest town. The mine will operate 350 days/year, 3 shifts/day, for a total mine life of 10 years.
Alaska Mine Models
The Interior Alaska mine is assumed to have the same basic pit layout as the Nevada mine model discussed above. Mine life will be the same, however, the mine will only operate 175 days/year, 2 shifts/day. The electrical rate is 0.09 $/kW·h and power is provided by on-site diesel generators. Power output will also be dependent upon daily tonnage. Camp accommodations for personnel are accounted for in infrastructure costs. Due to the variability of access distances in Alaska, road lengths and widths are assumed to be similar to the Nevada operation. Clearing costs per hectare (acre) are assumed to be higher than the Nevada model due to heavier vegetation. Drainage systems will likewise be increased over the Nevada model due to Alaska’s heavier precipitation and snow melt-off.
Nevada Mill Models
The heap leach portion of the Nevada mill model operates at the same rate the mine operates. After ore has been hauled from the pit, it is taken to the crusher circuit where run-of-mine ore is reduced to 1.27 cm (½ inch). The ore is then moved by conveyor to a cement silo where 5 kg of portland cement is added per mt (10 lb/st) ore. The ore/cement is then fed through a trommel agglomerator for mixing. There a cyanide solution is sprayed over the material, resulting in a moisture content of approximately 12%. Application of cyanide solution instead of ordinary water gives the leaching process an earlier start than conventional drip-line application alone.
After passing through the trommel, the ore is moved by a slightly inclined conveyor to radial stacking units. As described earlier, the tumbling action of falling from the end of the stacker to the heap pad is sufficient for agglomeration with minimal compaction.
The heap leach pad consists of a lower liner of 8 mil reinforced polyethylene over a base of compacted, rock-free soil. A 30 cm (12 inch) layer of soil is installed between the lower liner and a 30 mil reinforced polyethylene upper liner. With regular sampling, this soil layer serves as the leak detection unit. Finally a 60 cm (24 inch) layer of sand is placed on the upper liner to protect it from tearing during ore emplacement. Perforated polyvinyl chloride (PVC) piping with holes drilled at 5 cm (2 inch) spacing will be installed in this sand layer for solution collection. Pads are equipped with piezometers to monitor leakage between the liners. Pregnant and barren ponds are lined with 60 mil HDPE geomembrane.
After emplacement on the leach pads, the agglomerated is given 48 hours to cure. Heap lift heights do not exceed 3 m (10 ft). After the first lift is leached, a second lift of the same height is added to the top. Drip emitters are installed 1 m (3 ft) beneath the surface of the heap. Burial provides protection from evaporation and freezing.
Cyanide solution is be added to the heaps at a rate of 0.002 to 0.003 l/s/m² (0.003 to 0.005 gpm/ft²). The solution is collected in ponds and pumped to the mill building, where the precious metals are extracted by a carbon-in-column plant. Loaded carbon is stripped with a hot caustic cyanide solution. This solution is then electrolyzed onto steel wool and smelted on site for a final product of gold dore.
Alaska Mill Models
The Alaskan mill mode! utilizes the Nevada mill layout described above. The mill operates, however, only 175 days/year, 2 shifts/day. Yearly tonnage rates for each mine type is detailed in Table 3. The amount of water reclamation equipment and embankments constructed for ponds are greater than in Nevada, due to heavier precipitation and snow-melt runoff. The likelihood of flooding is consequently higher for the Alaska mine. As a result, the pond size will need to be larger to contain diluted cyanide solution.
Recoverable Metal Value
Once capital and operating costs were determined for each mine tonnage rate, the results were entered into a cash flow analysis computer program. A sensitivity analysis was performed to determine the recoverable metal value (RMV) which caused the cash flow analysis to yield a 0% and 15% discounted cash flow rate of return on investment (DCFROR). The RMV is the monetary value of the metal recovered from each ton of a mineral deposit. Use of this variable eliminates the effects of variable metal prices, grade, and heap leach recoveries. The RMV which yields a 0% DCFROR is the amount a deposit would need to recover to break-even and achieve zero profit during the course of its life. An RMV which yields a 15% DCFROR is the value needed for the deposit to be considered economic.
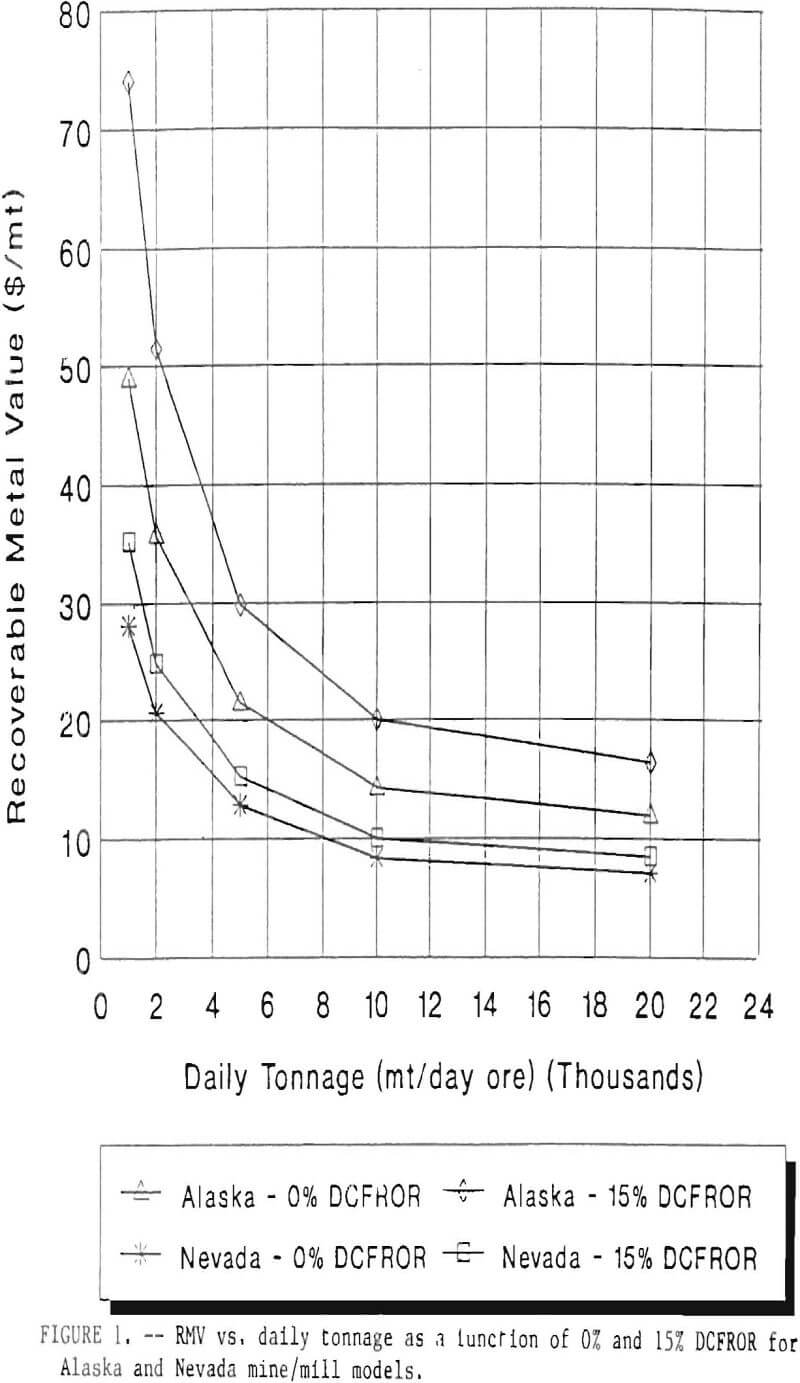
RMV is plotted versus daily production tonnage in figure 1. The Alaska models and the Nevada models each generated two curves at 0% and 15% DCFROR. An analysis of the RMV for each mine type, detailed in Table 3, reveals two multipliers: at 0% DCFROR, Alaskan RMV’s are 1.71 times greater than those of Nevada mines of similar tonnage; and at 15% DCFROR, Alaskan deposits require 2.01 times the RMV of similar Nevada deposits.
Summary
Based on figure 1 and Table 3, an Alaskan precious metal deposit that would be amenable to cyanide heap leaching requires an RMV 1.71 times greater than those of similar deposits in Nevada at the break-even level of probability. For a 15% DCFROR, an Alaskan deposit requires an RMV 2.01 times greater than those in Nevada. This increase in costs is due to the short leaching season and increased costs of transportation, shipping, and labor. Environmental factors also contribute to greater costs in Alaska due to such factors as Alaska’s heavier precipitation rate, permafrost, and vegetation cover. As a consequence, containment of effluent during flood events will cost more in Alaska due to the necessity for larger holding ponds.
A problem unique to Alaska heap leach operations is permafrost. If possible, pads should be placed on thaw-stable ground. In the event that this is not possible, all effort must be taken to keep the ground beneath the pad frozen. This can be accomplished through the use of thaw-pipes, or insulating gravel bases. Because the heap itself will provide insulation for the heaps (unless the leach solution is heated), the perimeter around the heap will be most likely to thaw. If this happens, the pad may be torn, solution may escape, or the edge of the heap may collapse.
Based upon the success of the Ryan Lode operation near Fairbanks, heap leaching is economically viable in Alaska. There are a few problems unique to northern heap leaching, such as short leach season, snow melt, and permafrost, which may be resolved by further research. Bureau research currently includes biotechnology to enhance refractory ore recovery and to reduce cyanide mine wastes. The Bureau is also working on a mill processing/leach practices study report and a leach reclamation handbook.
Appendix – Summary of Capital and Operating Costs for Alaska and Nevada Mine/Mill Models.
Costs for these models were estimated using the Bureau’s Cost Estimation System (CES), the Western Mine Engineering’s Mining Cost Service (MCS), and published case histories. The costs generated from these sources are based on establishing a mining operation in the western contiguous United States. For applicability to Alaska, escalation factors were used. Capital costs were multiplied by 1.52. Operating costs were multiplied by 1.16 for labor, and supplies and equipment by 1.52. All costs are expressed in July 1989 dollars.