Carbon and nitrogen, the two elements that make up cyanide, are present all around us. Together they make up almost 80% of the air we breathe, and both are present in the organic molecules that are the basis of all life forms. Hydrogen cyanide was formed in the earliest stages of the development of our planet as a precursor to amino acids, from which life on Earth evolved. Cyanide is formed naturally. It is produced and used by plants and animals as a protective mechanism that makes them an unattractive food source. Many organisms may either adapt to the presence of cyanide or detoxify it.
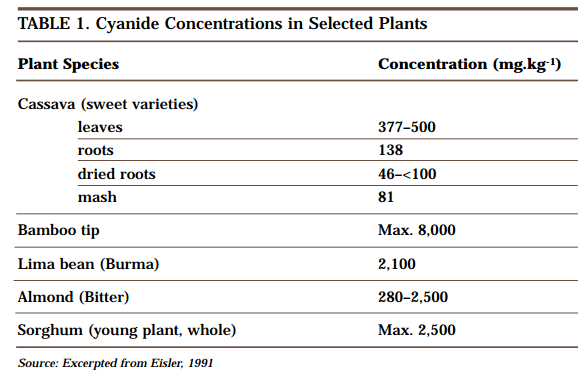
A natural source of hydrogen cyanide (HCN) is a sugar-like compound called amygdalin, which exists in many fruits, vegetables, seeds and nuts, including apricots, bean sprouts, cashews, cherries, chestnuts, corn, kidney beans, lentils, nectarines, peaches, peanuts, pecans, pistachios, potatoes, soybeans and walnuts. In the kernel of bitter almond, there is about 1 mg of HCN as amygdalin. Table 1 presents data on the amount of cyanide present in a variety of other foodstuffs.
Cyanide compounds are produced in thousands of plant species and in other life forms. In some plants, cyanide occurs in concentrations that would be judged “hazardous” if they were associated with manufactured sources. Plants such as alfalfa, sorghum and cassava are known sources of cyanide poisoning to livestock and humans. In addition to these naturally occurring forms of cyanide, cyanide compounds are also present in such everyday anthropogenic sources as automobile exhaust, cigarette smoke, and even road and table salt.
Cyanide is produced naturally in a number of microorganisms, insects and plants. Cyanide is a naturally occurring molecule of carbon and nitrogen. It existed on Earth before life began and was one of the fundamental building blocks in the evolution of life. Low concentrations of cyanide are present in nature, for example in many insects and plants, including a wide range of vegetables, fruits and nuts, where it provides protection against predators. In addition, cyanide is present in much of the everyday environment to which we are exposed, for example in road salt and automobile exhaust and as a stabilizer in table salt.