Oxidation potentials have been measured in the presence of various concentrations of cyanide, ferrocyanide, and ferricyanide and ethyl xanthate at various values of pH and related to flotation response. Eh-pH diagrams are constructed and show that the formation of surface ferric ferrocyanide is probably responsible for depression when cyanide is added.
Experimental Materials and Techniques
Pure potassium ethyl xanthate was used as collector, and reagent grade potassium cyanide, potassium ferrocyanide, potassium ferricyanide were used as depressants. Reagent grade HCl and KOH were added for pH adjustment. Conductivity water, made by passing distilled water through an ion exchange column, was used in all of the experimental work.
Two pure samples of pyrite were used in the investigation. Sample preparation for flotation involved dry-grinding with a mortar and pestle and sizing the product to 100 x 200 mesh. Prior to flotation, a 0.75-gram sample of the mineral was added to a solution containing a known amount of depressant at the desired pH value, and the system was conditioned for four minutes. Following this, a known amount of collector was added and the system was conditioned for another four minutes. The pH was measured, termed flotation pH; the pulp was transferred to a Hallimond cell, and flotation was effected with 50 cc of nitrogen for one and one-half minutes. The pH was also measured after flotation to ensure that no significant variation had occurred during the experiment.
Experimental Results
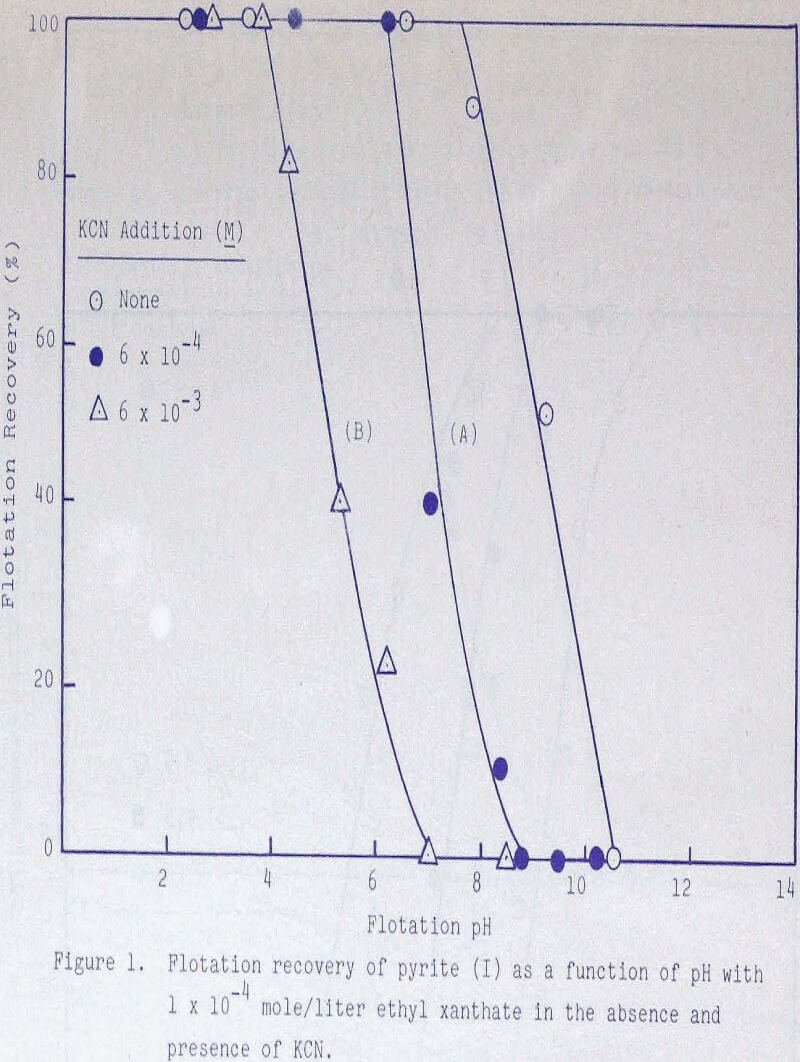
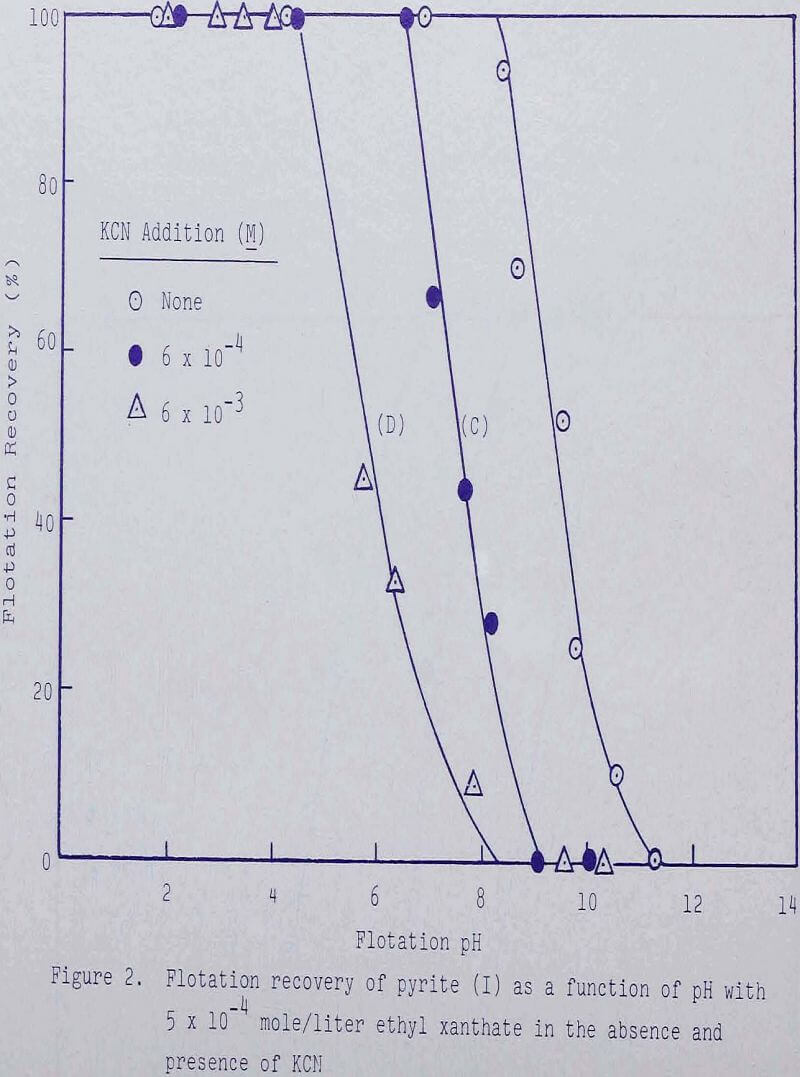
Experiments were conducted in the absence and presence of various amounts of potassium cyanide and ethyl xanthate to establish regions of flotation and depression. With 1 x 10 -4 mole/liter ethyl xanthate, complete flotation was effected until about pH 7-5- When 6 x 10 -4 mole/liter cyanide was added, complete flotation was obtained until about pH 6, while depression was noted at about pH 9. With 6 x 10-³ mole/liter cyanide, depression was noted to occur approximately two units in pH sooner. Increasing the collector addition to 5 x 10 -4 mole/liter resulted in increased recoveries at higher values of pH.
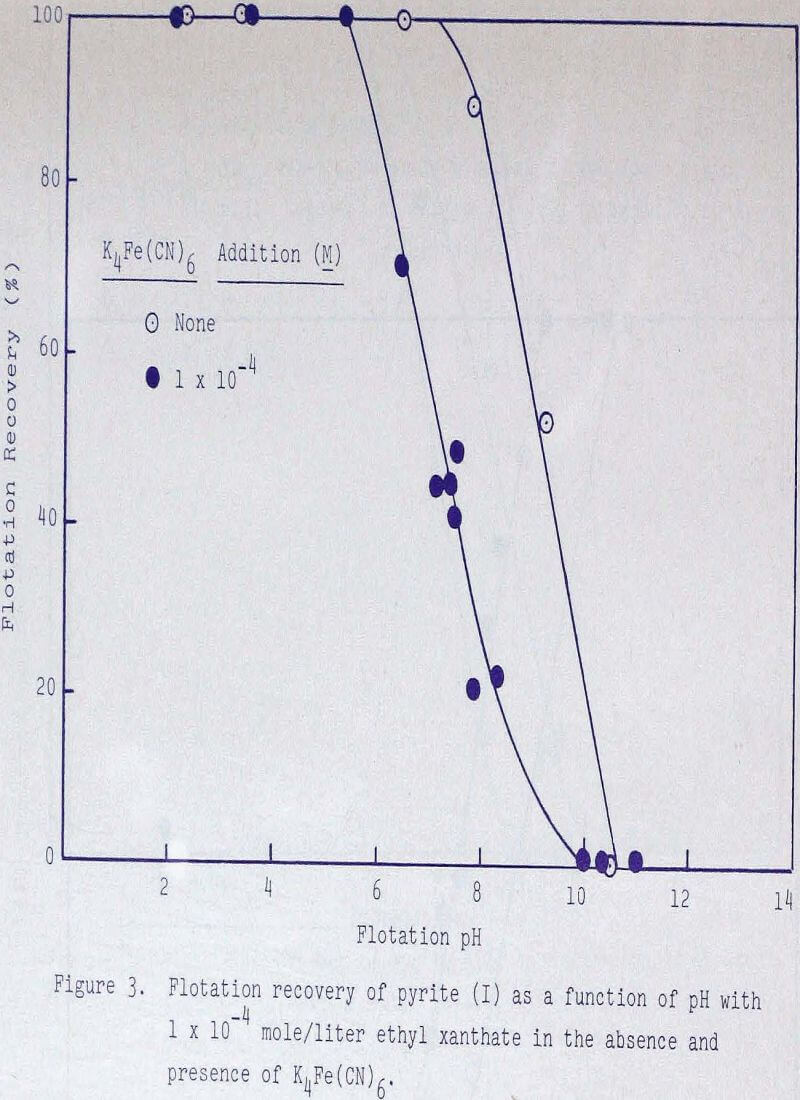
The depressing action of ferrocyanide and ferricyanide was also examined. As shown in Figures 1, 3, and 4, ferrocyanide is more effective than ferricyanide in depressing pyrite but functions very nearly the same as an equivalent amount of CN-.
In view of the fact that some authors have reported slight depressing effects with ferrocyanide, while others have reported the opposite, it seemed likely that these phenomena were related to differences in the pyrites used. As a result, another sample of pyrite was floated In the absence and presence of cyanide and ferrocyanide.
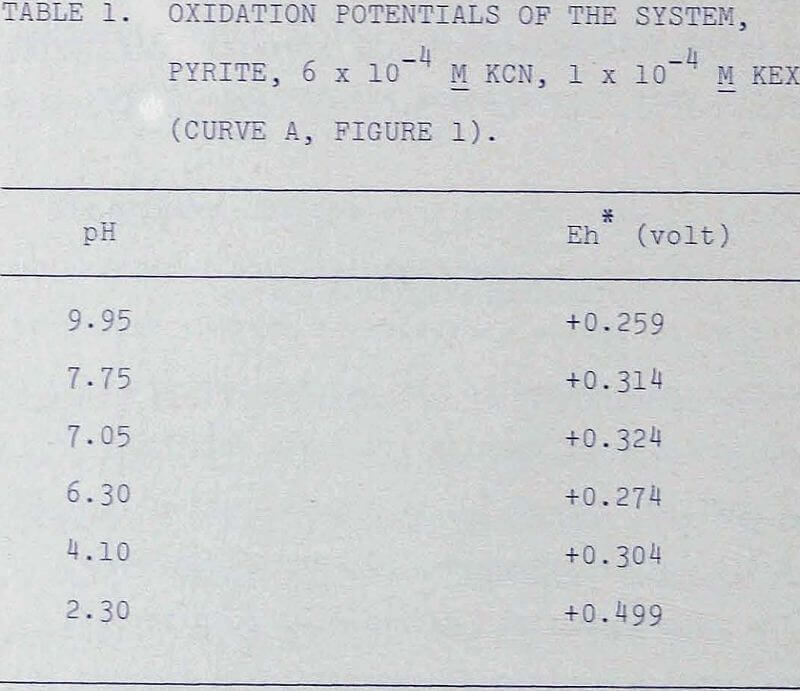
The oxidation potentials of the pyrite systems were also measured under various conditions and are listed in the following tables.
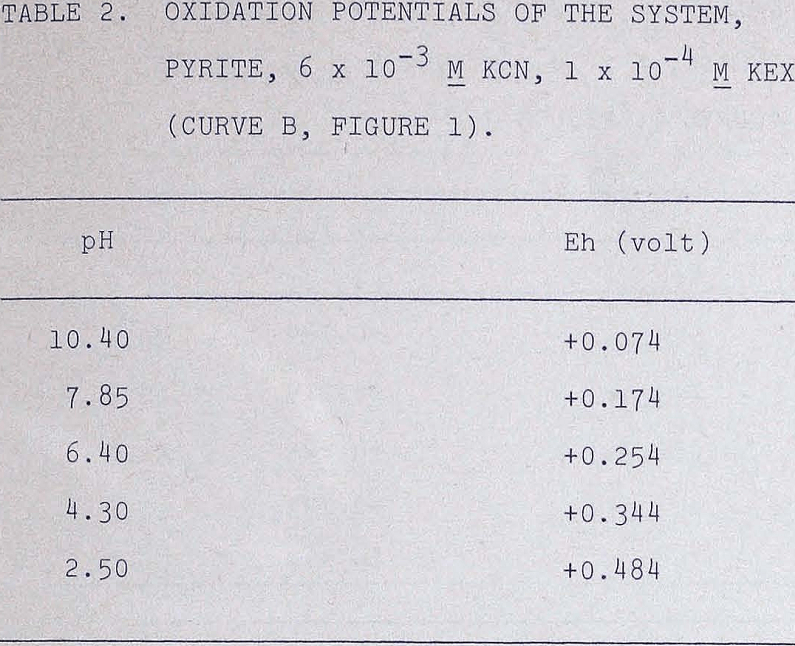
Discussion of Results
One of the objectives of this work is to establish which species in the pyrite-cyanide-xanthate system is responsible for depression and then the conditions under which this species will adsorb. The zeta potential results reported are quite informative in this regard. First, they show that ferrocyanide, ferricyanide and cyanide (or other cyanide species) adsorb chemically on pyrite. Secondly, it can be noted that the effects of these three reagents decrease with increasing values of pH, and finally at pH 10.5 and above, the zeta potentials of pyrite become solely dependent on the hydroxyl ion concentration.
At this stage of the discussion, it will be helpful to mention, briefly, the chemistry of ferrocyanide and ferricyanide ions and to discuss the zeta potentials obtained in their presence. Both ferrocyanide and ferricyanide ions are known to be inert compounds, i.e., they can remain in solution for hours without dissociation. Furthermore, it has been shown by radiometric analysis that there is no exchange between the iron of ferrocyanide and ferricyanide and the ferrous or ferric ions that may exist in solution.
Certain Eh and pH values must be satisfied before the formation of the compound Fe4 [Fe(CN)6]3 can occur, or stated in other words, certain Eh and pH values will have to be satisfied before depression of pyrite by cyanide can occur if the formation of surface ferric ferrocyanide is responsible for depression. It should be mentioned that Eq. 1 actually represents a two-step reaction. That is, ferrous ion, derived from the surface of pyrite, reacts with cyanide to form ferrocyanide ion, Fe(CN)6 -4, which in turn probably reacts with surface ferric ion to produce some form of the compound Fe4 [Fe(CN)6]3.