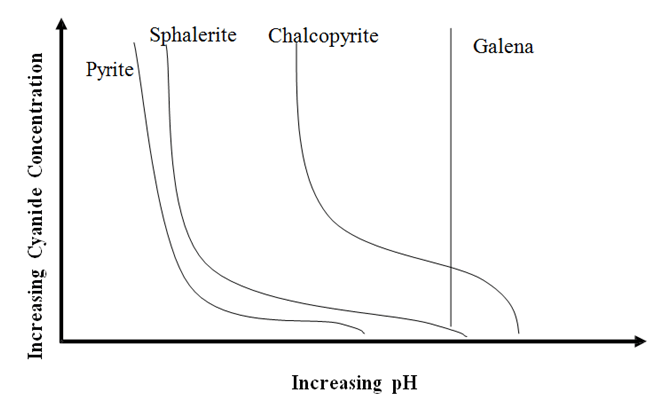
The chart displays how the Cyanide Concentration (how much NaCN) is needed to effectively Depress Sulphides like Pyrite, Sphalerite, Chalcopyrite but not Galena at various pH.
It is pH that affects/impact the required effective concentration of cyanide. The higher the pH, the less NaCN is needed as CaO/Lime also acts as a Sulphide depressant. The use of cyanide complements it will in froth flotation separation.
Using NaCN at the worn pH can be totally wasteful and ineffective as well as DANGEROUS!
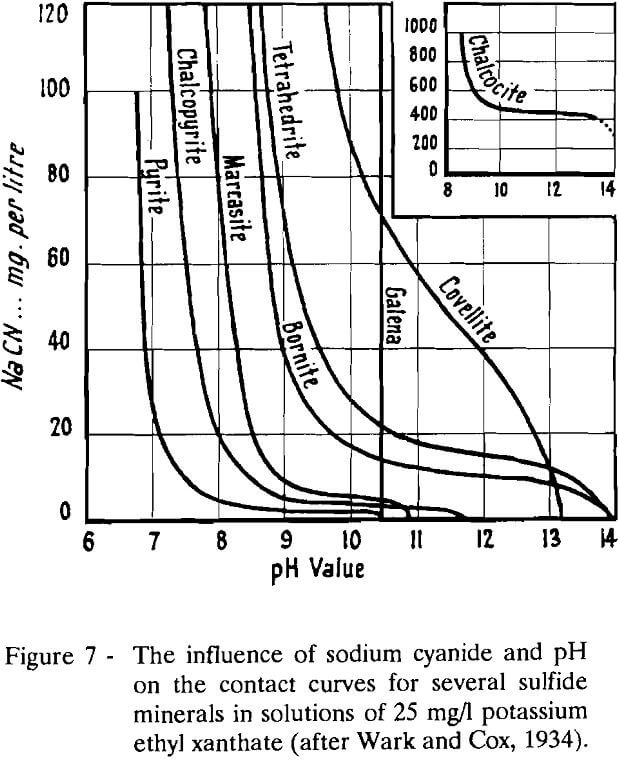
Using the captive bubble method to assess the combined influence of cyanide and alkalis on the flotation of sulfide minerals with thiol collectors, it was determine the conditions at which these agents function as depressants, which correspond to those where no contact is possible between the mineral and the air bubble. Their results for several minerals are presented in Fig. 7. In this figure, contact is possible at all pH values and concentrations below and to the left of the curves. According to these results, air would make contact with chalcopyrite, but not with pyrite, at a pH value of 7.5 and a sodium cyanide concentration of 30 mg/l. Under these same conditions, contact would be easily possible with the other copper minerals. In their theory of the action of cyanide, some postulated that the mechanisms of ion exchange and ion adsorption are responsible for the interaction of the mineral surface with chemical reagents in the pH region where ionic species predominate in solution.
