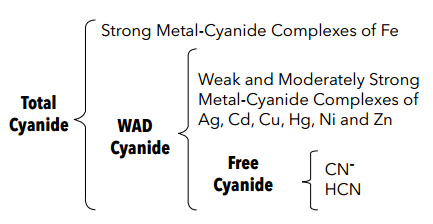
 Summary of CN Analysis Methods
Summary of CN Analysis Methods
|
Description Name |
Method | Description | Potential Interferences |
Measurement |
| Free Cyanide | ASTM D 4282 | Passive Diffusion at pH 6 and room temperature | • Storage
• Measurement |
• Manual chlorination of cyanide with chloramine-T and subsequent reaction with pyridine-barbituric acid.
• Maximum absorbance is determined by manual colorimetry. |
| ASTM D 7237 | Flow Injection Analysis (FIA) into a reagent at a pH of 6-8 | • Storage
• Measurement |
Gas Diffusion-Amperometry | |
| SM 4500-CN G | • Alkaline Chlorination to destroy all weak and dissociable metal-cyanide complexes and any free or simple cyanide.
• Two manual distillations (total-chlorinated)=CATC |
• Excessive light, | • Manual chlorination of cyanide with chloramine-T and subsequent reaction with pyridine-barbituric acid.
• Maximum absorbance is determined by manual colorimetry. |
|
| ASTM D2036 | ammonia.
• Incomplete recovery on Ag-cyanide complexes. • Storage • Distillation • Measurement |
|||
| Available Cyanide (CATC) (WAD) | ASTM D2036 | Buffered (pH 4.5) manual distillation | • Excess of Fe-cyanide complexes.
• Incomplete recovery on Hg-cyanide complexes. • Storage • Distillation • Measurement |
• Manual chlorination of cyanide with chloramine-T and subsequent reaction with pyridine-barbituric acid.
• Maximum absorbance is determined by manual colorimetry, Manual Ion Selective Electrode (ISE), Titration. |
| ASTM D4374 | Buffered (pH 4.5) automated flash distillation | • Storage
• Distillation • Measurement |
• Automated chlorimation of cyanide with chloramine-T and subsequent reaction with pyridine-barbituric acid.
• Automated colorimetry |
|
| Kelada 01 | ||||
| OLA 1677 ASTM D6888 | Pretreatment with ligand exchange reagents, room temperature automated gas diffusion. | • Storage
• Measurement |
Amperometry | |
| SM 4500 CN D | • Cyanide ion is released from cyanide complexes as HCN by means of macro manual reflux- distillation in the presence of a strong sulfuric acid solution and magnesium chloride.
• The HCN is swept into a dilute sodium hydroxide absorber solution. |
• Storage
• Distillation • Measurement • Excessive Light |
Cyanide in the absorber solution is titrated with silver nitrate. | |
| SM 4500 CN E | • Cyanide in the absorber solution is converted to cyanogen chloride by manual reaction with chloramine-T at pH <8.
• After reaction, the cyanogen chloride forms a red-blue color on addition of pyridine- barbituric acid reagent. • Maximum color is determined by manual colorimetry. |
|||
| ASTM D2036 | • Cyanide ion is released from cyanide complexes as HCN by means of a macro, or scaled down manual reflux-distillation in the presence of a strong sulfuric acid solution and magnesium Chloride.
• The HCN is swept into a dilute sodium hydroxide absorber solution. |
• Storage
• Distillation • Measurement • Excessive Light |
• Cyanide in the absorber solution is titrated with silver nitrate.
• Cyanide in the absorber solution is determined manually by Ion Selective Elextrode (ISE). • Cyanide in the absorber solution is converted to cyanogen chloride by manual reaction with chloramine-T at pH <8. • After reaction, the cyanogen chloride forms a red-blue color on addition of a pyridine- barbituric acid reagent. • Maximum color is determined by manual colorimetry. |
|
| Total Cyanide | EPA 335.4 | • Cyanide ion is released from cyanide complexes by means of a scaled down (midi) manual reflux-distillation in the presence of a strong sulfuric acid solution and magnesium chloride.
• The HCN is swept into a dilute sodium hydroxide absorber solution. |
• Storage
• Distillation • Measurement • Excessive Light |
• Cyanide in the absorber solution is converted to cyanogen chloride by automated reaction with chloramine-T at pH <8. After reaction the cyanogen chloride forms a red-blue color on addition of a pyridine- barbituric acid reagent.
• Maximum color is determined by automated colorimetry. |
| Kelada 01, ASTM D4374, EPA 335.3 | • Cyanide ion is released from cyanide complexes by means of UV irradiation and automated flash distillation in the presence of a strong sulfuric acid solution.
• The HCN generated is injected into an auto chemistry analyzer chemistry cartridge. |
• Storage
• UV Irradiation • Distillation • Measurement |
• Cyanide in the adsorber solution is converted to cyanogen chloride by automated reaction with chloramine-T at pH <8.
• After reaction, the cyanogen forms a red- blue color on addition of a pyridine- barbituric acid reagent. • Maximum color is determined by automated colorimetry. |
|
| ASTM D7284 | • Cyanide ion is released from cyanide complexes by means of a scaled down (midi) manual reflux-distilation in the presence of a strong sulfuric acid solution and magnesium chloride.
• The HCN is swept into a dilute sodium hydroxide absorber solution. |
• Storage
• Distillation • Measurement |
Cyanide in the absorber solution is determined by automated gas diffusion amperometry. | |
| OIA1678/ASTM D7511-09 | • Cyanide ion is released from cyanide complexes by UV irradiation.
• The HCN generated diffuses across a membrane and is measured amperometrically. |
• Storage
• UV Irradiation • Measurement |
Cyanide is determined by automated gas diffusion amperometry. |
Commonly Used Cyanide Analysis Methods
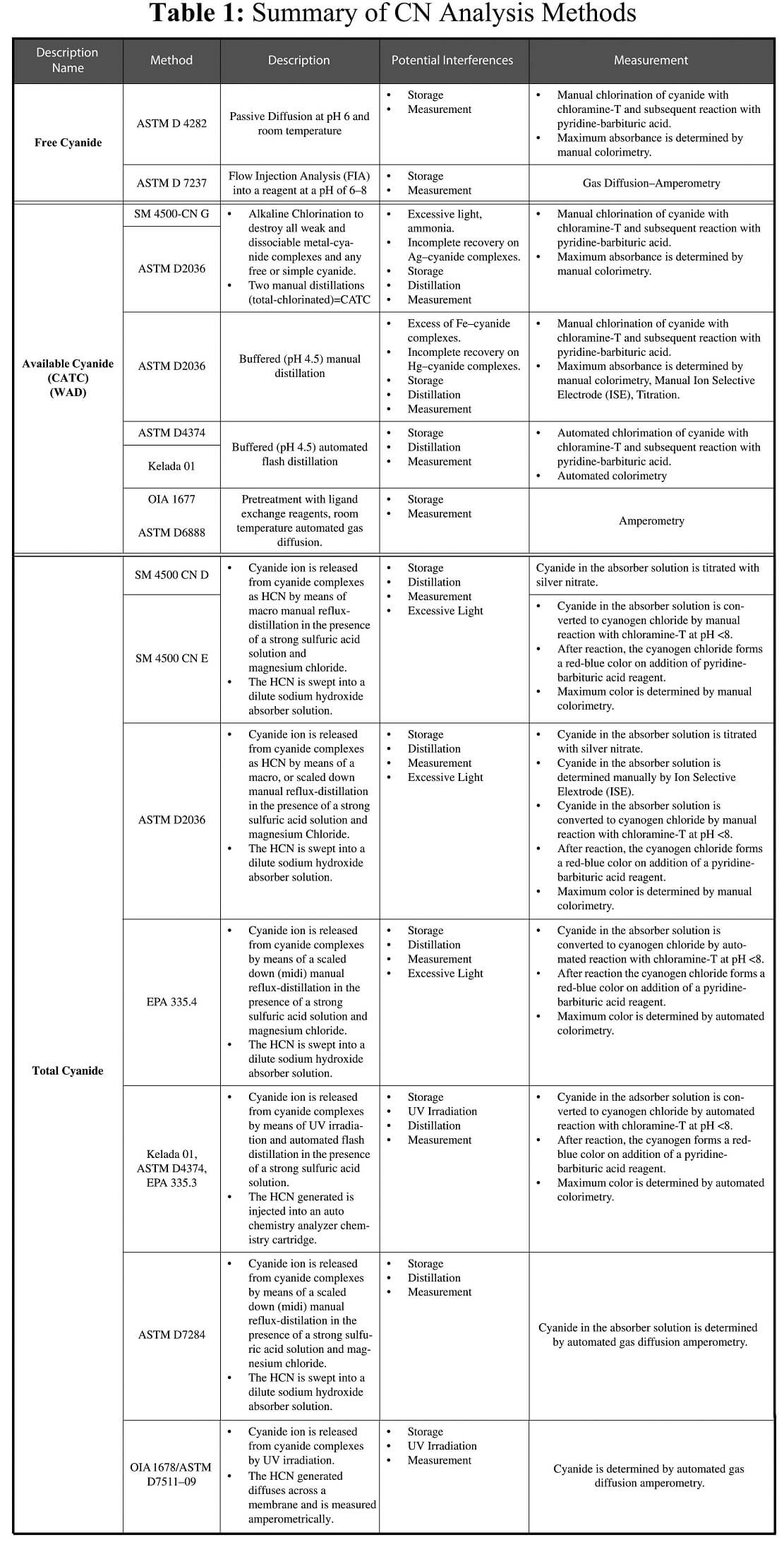
Free Cyanide
Free cyanide refers to the sum of hydrogen cyanide (HCN) and cyanide ion (CN-) in a sample. Free cyanide is bioavailable and approximately a thousand times more toxic to aquatic organisms than it is to humans.
Analytically, free cyanide is referred to as the amount of HCN liberated from solution at pH 6.0.
Weak to Moderately Strong Metal – Cyanide Complexes
Weak to moderately strong metal-cyanide complexes are compounds that dissociate and release hydrogen cyanide gas under mildly acidic conditions (pH 3 to 6). Cyanide species within thiscategory include:simple cyanides–soluble/dissociable alkali metal and alkali earth metal-cyanide complexes (NaCN, KCN, Ca(CN)2); weak metalcyanide complexes (Zn(CN)42-, Cd(CN)3-); and moderately strong metal-cyanide complexes (Cu(CN)2-, Ni(CN)
42-,Ag(CN)2-). Weak Acid Dissociable (WAD), Cyanide Amenableto Chlorination (CATC), and Ligand Exchange methods were developed to quantify the sum of these cyanide species as well as any free cyanide present in a sample.
Methods intended to measure weak to moderately strong metal–cyanide complexes also measure simple cyanides.
Simple cyanides include free cyanide, alkali metal cyanides, alkali earth metal cyanides, and ammonium cyanide.
Strong Metal-Cyanide Complexes
Strong metal-cyanide complexes are compounds that require strongly acidic conditions (pH < 2) to dissociate and release hydrogen cyanide gas. Examples of strong metal-cyanide complexes include Fe(CN)62-, Fe(CN)64-,Co(CN)64-, and Au(CN)2-. The strong acidic conditions used to dissociate these resistant metal-cyanide complexes readily dissociates all other cyanide species present in a sample. “Total Cyanide” is the term used in U.S. EPA methods to refer to the sum of all cyanide species that are converted to hydrogen cyanide following reflux distillation of a sample acid in a strong solution.
Analytical Methods of Cyanide
Total Cyanide: Measured by Reflux Mineral Acid distillation Method.
•Includes Includes complex complex iron‐cyanide cyanide, WAD, free cyanide cyanide, and other inorganic complexes
Amenable Cyanide: Difference between total CN before and after chlorination
Weak‐Acid‐Dissociable Cyanide (WAD): Distillation method as Total Cyanide but weak acid is used.
• Includes Includes CN ion, HCN, and some complexes complexes (cadmium (cadmium, copper, nickel, silver, and zinc)
Free Cyanide: Titration with AgNO3, Ion Specific Electrode, Solvent extraction or sparging HCN and collecting it for b d l pgg g subsequent cyanide analysis.
• Includes CN‐ and HCN
