Clennell gives among other cyanate assay methods the following based on the fact that when KCNO is treated with an acid it does not decompose into HCN but reacts in the sense of the equation,
KCNO + 2HCl + H2O = KCl + NH4Cl + CO2
By determining the amount of ammonium compound formed a measure of the cyanate originally present is obtained.
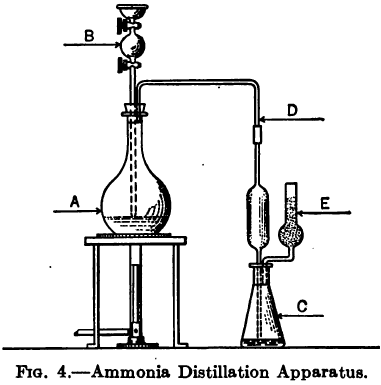
The accompanying diagram, Fig. 4, shows the general arrangement of apparatus recommended by Sutton for the distillation of ammonia.
A is a distilling flask holding 200 or 300 cc fitted with a rubber stopper having two holes for glass tubes to pass through it.
B is a bulb tube with glass stopper above and stopcock below the bulb.
C is the receiver with rubber stopper through which the distillate tube D enters, and another tube E for escape of superfluous gases. The tube E is partly filled with glass beads or glass wool. In order to prevent the possibility of spray entering the distillate tube from the boiling liquid it is well to place a plug of glass wool in the neck of flask A just below the end of the distillate tube.
The distilling tube D should extend down almost to but not into the acid. Sutton recommends that both ends of the distillate tube and that of the vent tube E be ground obliquely. Should the distillate flask get too hot it may be stood in a dish of water, and a little water played over it at intervals with a wash bottle.
Cyanate Assay Determination Method and Procedure
Take a measured amount of the solution to be tested and add HCl in slight excess: evaporate to dryness over the water bath: dissolve the residue in water and transfer to the boiling flask A. Place 20 to 30 cc of strong NaOH solution in the bulb B and stopper the flask carefully. Loosen the stopper of flask C and pour in through the glass wool of tube E a measured quantity of decinormal acid. (The quantity of acid should of course be more than sufficient to combine with the whole amount of ammonia given off.) A few drops of methyl orange may be added if desired.
All being ready the stopcock in tube B is opened and the caustic solution allowed to run into flask A, which is then brought to a gentle boil. Boiling should be continued till at least two-thirds of the liquid in the flask has distilled over. When the operation is finished disconnect flask C and wash down the acid solution contained in the glass wool of tube E into the flask, great care being taken that every trace of acid is displaced. The contents of flask C are then titrated with decinormal alkali to ascertain the quantity of acid neutralized by the ammonia.
1 cc N/10 acid consumed = 0.0042 gm. CNO
Should ammonium compounds be present in the solution to be tested they must of course be estimated separately and their equivalent deducted from the value found before calculating the result to terms of CNO.
