In copper SX processes, the copper extracted from the aqueous feed solution into the solvent is replaced by hydrogen ions given up by the solvent. The aqueous therefore becomes more acidic as it loses copper. To reclaim (strip) the copper from the solvent, this reaction is reversed by contacting the solvent with a more highly acidic solution. The reversible reaction is written in the following form, somewhat oversimplified but descriptive and useful:
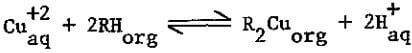
where R is extracting reagent molecule without proton, and subscripts denote aqueous and organic phases.
For a given extracting reagent there are practical limits to the acidity and copper content of a feed solution from which economic recovery can be made. Other constituents of the feed solution can also negatively affect extraction, such as chloride ions, which complex cupric ions. These limitations on feed solutions are relieved with more powerful extractants, but such extractants require more acidic solutions for effective stripping. If cathode copper is to be electrowon from these solutions, an upper limit on acidity is imposed by the corrosive attack of sulfuric acid on the lead alloy anodes conventionally used in EW. This limit is generally conceded to be in the range of 175 to 200 grams free sulfuric acid per liter.
LIX 64N is a reagent widely used to extract copper from acidic feed solutions of pH 1.7 to 2.5 containing 1 to 5 grams copper per liter, originating from dump leaching or from agitated leaching and countercurrent decantation. This reagent is effectively stripped by solutions containing 100 to 160 gpl free sulfuric acid per liter. LIX 605 is a more acidic reagent and has demonstrated effective extraction from vat leach liquors in the neighborhood of 10 grams per liter each copper and free sulfuric acid. However, stripping solutions for such reagents are at or near the acidity limit for EW.
LIX 70 is a very powerful copper extractant capable of economic recovery from yet more difficult feed solutions. These might include higher tenor vat leach liquors or liquors from sulfide concentrate leaching 1) after roasting, 2) in autoclaves with oxygen and sulfuric acid, 3) in ferric sulfate solutions, or 4) in ferric chloride solutions. Unfortunately, effective stripping of this reagent requires 300 to 400 grams free sulfuric acid per liter. Crystallization would provide for the separation of copper sulfate from the acid present in the strip liquor, thus allowing cathode copper production by EW. Figure 1 and Table I describe this possibility. The flowsheet basis is 20 tons cathode copper per day.
The LIX 70 stripping circuit would be run at a controlled 40°C. The strip liquor composition indicated is saturated at 35°C, giving a 5°C safety margin. The copper/iron extraction ratio assumed is 500/l, which is conservative for this extremely selective reagent but will vary with feed solution composition and extraction conditions.
The crystallizer system cost has been estimated, by a major supplier of this type of equipment, at $500,000. This includes the crystallizer, recirculating leg with pump and heat exchanger, steam jet ejector, barometric condenser, hot condensate well with pump, interconnecting piping, construction of 316 and 316L stainless steels, and rudimentary temperature-pressure-density instrumentation, but does not include installation.
Assuming a steam cost of $5.25 per ton, the steam cost per pound of cathode copper is 1.97c.
The crystallizer temperature and pressure in this system would be 20°C and 11.6 mm Hg, respectively. The condenser would operate at 35°C and 41.1 mm Hg. Because of the steam input required for the pressure boost, the condensation heat exceeds the evaporation heat required in the crystallizer. Excess heat may be used to partially reheat the stripping solution returning to SX, although this circulation system and heat exchanger are not shown on the flowsheet. This system is not necessarily optimal for energy conservation. A freon-cycle heat pump might be considered, operating a refrigerated indirect condenser at the same pressure as the crystallizer, and again supplying more heat than required by evaporation in the crystallizer.
Very low iron content in this electrolyte should allow very high EW current efficiencies and freedom from cathode hanger loop corrosion problems.

