Table of Contents
CuSO4 Crystallization for Typically Acidic Extraction: A small body of acid-leachable copper ore or a small existing stream of copper-bearing acidic mine water is often unable to justify a plant to produce cathode copper. Electrowinning is capital intensive, and small reserves are generally considered higher risks for financing. Electrowinning is also intensive in electric power and labor, both of which are in short supply to
small operations in remote locations. The market for cathode is a large one, and small quantities may not bring the full market price. Many operators stay with the cementation process for these reasons, paying high prices for iron and getting low prices for cement copper, despite the feasibility and attractiveness of SX on a small scale.
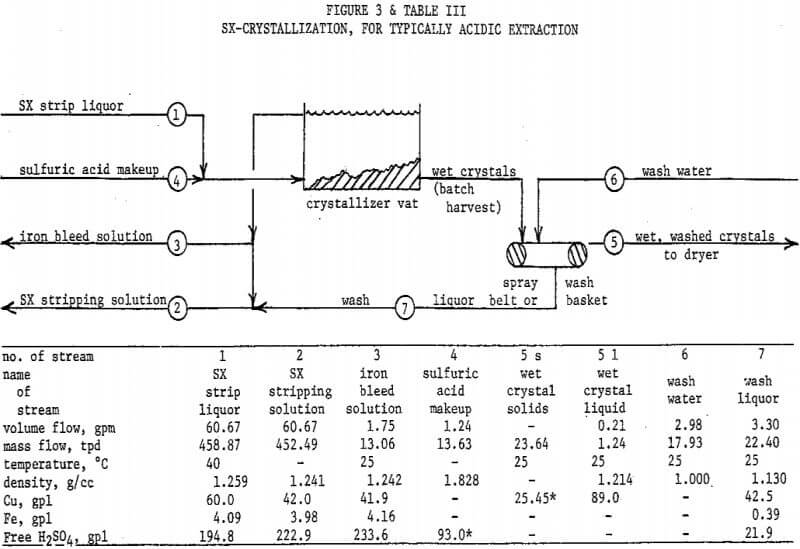
Production of copper sulfate as an end product is an alternative. SX strip liquor is a good feed to crystallization, which may be accomplished in simple vats. Figure 3 and Table III describe this possibility. The flowsheet basis is 24 tons per day of copper sulfate pentahydrate. The SX stripping solution and strip liquor are appropriate for LIX 64N, or a somewhat stronger (more acidic) extractant if required. The copper/iron extraction ratio assumed is 150/l.
The flowsheet assumes SX stripping to be operating at 40 °C, producing a liquor saturated at 35 °C, and uses a 25 °C crystallization temperature in the vats. Vats could be in an evaporatively cooled building or jacketed with evaporatively cooled water. The flowsheet could as easily be designed for a colder climate (or season), with SX maintained at 20-25°C and the vats allowed to approach a colder ambient.
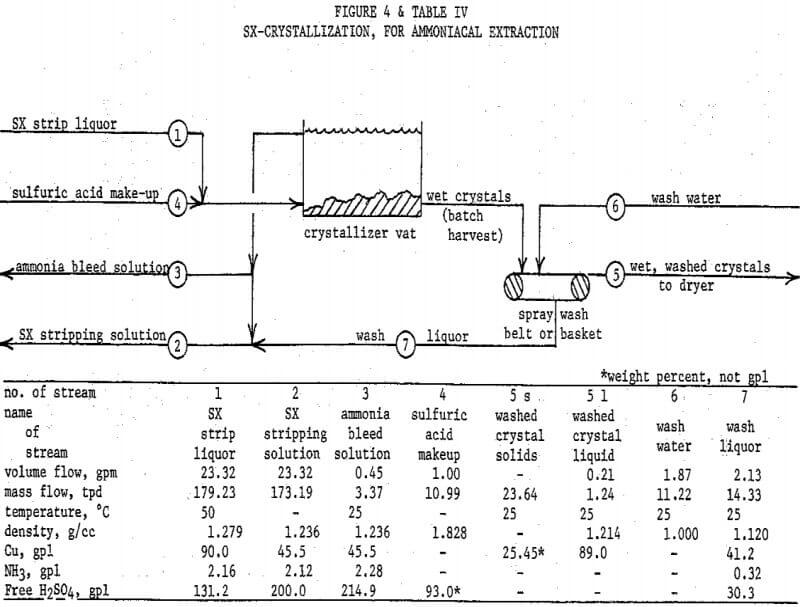
Ferric iron is not soluble in alkaline ammonia solutions, and ferrous iron is not common due to the oxidizing nature of the leach. Often the most troublesome co-extracted impurity is the ammonia, which must be controlled at a manageable level in the stripping solution by a bleed-off. This problem is minimal for LIX 54, and is further minimized by proper scrubbing of the copper loaded solvent with a near neutral solution before stripping. The copper/ammonia SX transfer ratio (after scrubbing) assumed in the flowsheet is 1000/l.
Compositions of Saturated Solutions
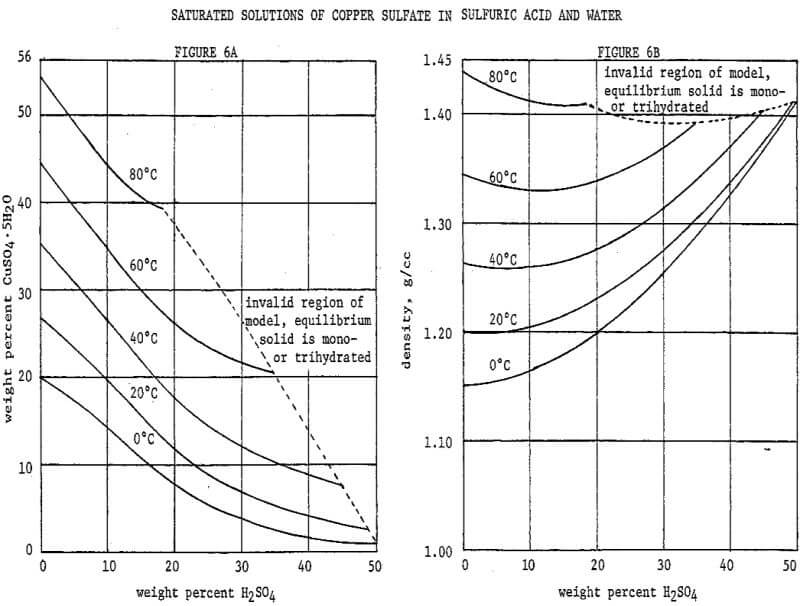
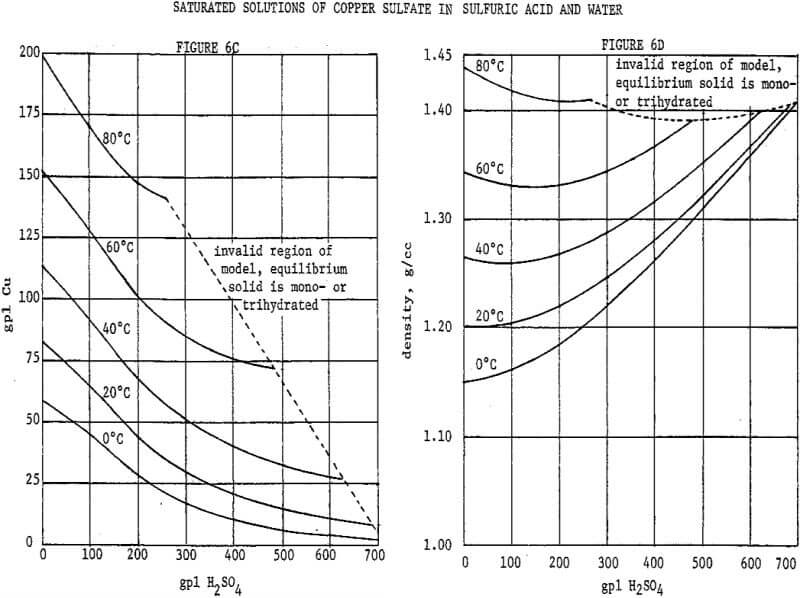
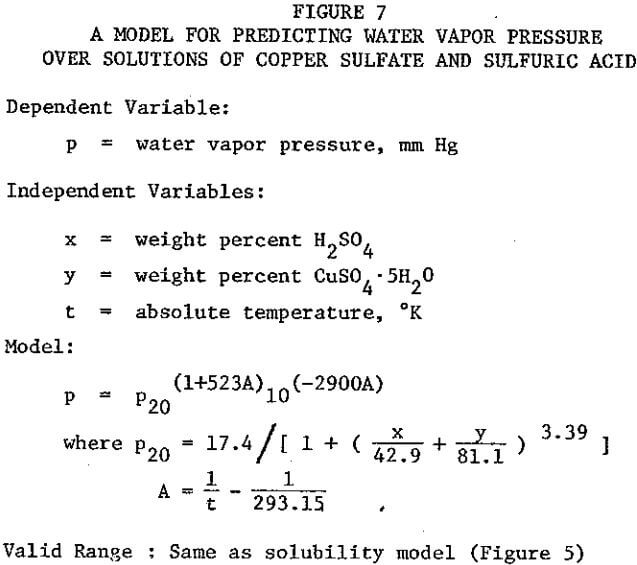
A reasonably optimal flowsheet and control scheme for a crystallization circuit require a convenient means of predicting the copper contents and densities of saturated solutions. As dependent variables these are related nonlinearly to two independent variables, temperature and acid content. Data are available in the literature, but linear interpolation of nonlinear data in two dimensions is cumbersome and inaccurate. The 1926-1927 data of Agde & Barkholt as reported in Linke were fitted with empirical mathematical models by multiple nonlinear least-squares regression. The author has devised new models, given in Figure 5, which are improved representations of the same data.
Referring to Figure 5 it will be noted that copper sulfate solubility, although referred to as a dependent variable, is an implicit function in the model. An iterative technique is therefore necessary to evaluate solubility. Temperature and acid content are referred to as independent variables, but either may likewise be treated as a dependent variable and evaluated by an iterative technique. Such techniques may be easily programmed on a computer, or advanced programmable calculator. The density model may be incorporated within the iterative loops, for operation of a program in the concentration mode as opposed to the weight percent mode. These techniques were used by the author in developing the flowsheets presented herein. However, in the interest of comprehension of the scope of the models, they are presented graphically in Figures 6A through 6D.
The presence of sulfate salts other than copper, although at levels well below their own saturations, will depress copper sulfate solubility in much the same way as does the sulfuric acid. The chief impurities found in copper SX strip liquors are ferric sulfate (from acidic extractions) and ammonium sulfate (from ammoniacal extractions). Where either is indicated on a flowsheet, its effect on copper solubility is accounted for by treating it as additional sulfuric acid at an equivalent normality. At the relatively low impurity concentrations indicated, these should be reasonable approximations.

CuSO4 Copper Sulphate Crystallization
Anhydrous CuSO4, isostructural with orthorhombic ZnSO4, is of particular interest because of its antiferromagnetic properties at low temperatures. Fundamental investigations on this compound would be greatly enhanced if single crystal specimens were readily available. Kokkoros and Rentzeperis obtained CuSO4 single crystals up to 1 mm in length by evaporation of an aqueous solution obtained by dissolving CuSO4-5H2O in water and adding H2SO4. Conditions under which the anhydrous salt may be obtained from an aqueous solution have been described in the early literature. Kreines, in an effort to prepare crystal specimens of this salt for magnetic susceptibility and anisotropy studies, dissolved CuSO4 in molten (NH4)2SO4; then, by controlling the rate of decomposition of the solvent, he was able to obtain single crystals of CuSO4 weighing up to 2 mg. Like many other sulfates, CuSO4 undergoes decomposition before the melting point is reached so that growth from the melt under normal laboratory conditions is precluded. The use of a nonaqueous solvent appears to be the most promising approach, and experiments in our laboratory indicate that a sulfuric acid-ammonium sulfate mixture offers some definite advantages over the single components as a solvent for CuSO4 single crystal growth.
Experimental Results and Discussion
Crystallization of Anhydrous Copper Sulfate
from Sulfuric Acid—Ammonium Sulfate Mixtures
Starting reagents used throughout this work were Baker “Analyzed Reagent” grade. The CuSO4·5H2O was further purified by recrystallization from distilled and demineralized water, then dehydrated by heating in a muffle furnace at 350 °C for 24 hr under a dry nitrogen atmosphere. The anhydrous powdered salt was stored in a dessicator over phosphorous pentoxide.
The sulfuric acid was adjusted to 100 percent composition by adding fuming sulfuric acid to the commercial 96 percent reagent, the freezing point method being used to determine when the 100 percent composition point was reached.
Solvents of various compositions were then prepared by heating a measured quantity of H2SO4 to 150 °C and adding a weighed amount of (NH4)2SO4 to give the desired composition.
The solubility of CuSO4 in solvents of varying (NH4)2SO4-H2SO4 ratios, at 200 °C, is indicated in figure 1. The experimental points were determined by adding powdered CuSO4 to the solvent, maintaining the temperature at 200 °C ±2 °C for 24 hr to assure equilibrium, sampling the solution, and determining the copper content and sulfate content iodometrically and gravimetrically, respectively. The sulfate analysis was corrected for the amount of sulfate present as CuSO4 so that the ordinates of figures 1 and 2 show the ratio of copper to solvent sulfate in the solution.
As figure 1 indicates, the solubility of CuSO4 in pure H2SO4 is relatively low, but increases rapidly as the (NH4)2SO4 content of the solvent increases. However, increasing the (NH4)2SO4 ratio also increases the viscosity of the solvent at any given temperature, so that higher temperatures are necessary to maintain the solvent in a fluid state. Practical working temperatures are limited by the fact that (NH4)2SO4 undergoes considerable decomposition above 300 °C. Since crystal growth is dependent on diffusion through the solution and since higher viscosities affect diffusion adversely, solvents of higher mole ratio than 0.35 (NH4)2SO4 were not considered.
The temperature dependence of CuSO4 solubility in 0.35(NH4)2SO4-0.65H2SO4 is shown in figure 2. In determining the experimental points, the temperature of the solution was controlled at the given value ±2 °C and maintained for 24 hr with occasional stirrings before sampling. A study of the copper content of the solution as a function of time, at the lowest temperature shown in figure 2, indicated that 24 hr was an adequate period to achieve equilibrium. Analyses for copper and sulfate were made as indicated above.


To carry out the crystal growth, approximately 500 ml of solution were placed in a large borosilicate glass test tube, heated by a conventional hot plate. An excess of CuSO4 powder was added to the solution, and a tantalum sheet, rotating at 10 rpm, was suspended in the solution near the surface. The test tube was loosely covered. The solution temperature was approximately 200 °C with a temperature gradient between the bottom of the container and the tantalum sheet of approximately 5 °C. Single crystals of CuSO4 grew predominantly at the edges of the tantalum sheet, but occasionally also on the flat surfaces. In three or four days, crystals weighing up to 150 mg have been obtained. Excess solvent was removed from the crystal surfaces by washing in 100 percent H2SO4 and heating to 400 °C.
Figure 3 is a photograph of several CuSO4 crystals. The specimens, which were transparent with a slight greenish tinge, were verified to be single crystals by Laue backscatter x-ray diffraction. Chemical analysis of the crystals indicated a composition of 99.7 percent CuSO4.
