Table of Contents
The process is as follows:—The cupels, which should have been made some time before and stored in a dry place, are first cleaned by gentle rubbing with the finger and blowing off the loose dust; and then placed in a hot muffle and heated to redness for from 5 to 10 minutes before the alloy to be cupelled is placed on them. The reasons for this are sufficiently obvious: the sudden evolution of much steam will blow a cupel to pieces ; and, if the whole of the water has not been removed before the cupel is filled with molten lead, the escaping steam will bubble through, and scatter about particles of the metal. If some particles of unburnt carbon remain in the bone ash, a similar result will be produced by the escape of bubbles of carbonic acid as soon as the fused litharge comes in contact with them. The cupels having been prepared are arranged in a definite order in the muffle, and the assay but tons are arranged in a corresponding order on some suitable tray (cupel tray, fig. 41); the heat of the muffle being at bright redness.
Then with the help of the tongs (fig. 42) the assay buttons should be placed each in its proper cupel ; a note having been previously made of the position it is to occupy, and the door of the muffle closed.

This part of the work should be done promptly, so as not to unduly cool the muffle : the start requires
a fairly high temperature, and is a critical part of the process. A black crust forms at once on the surface of the lead ; but this ought soon to fuse and flow in greasy drops from off the face of the metal, so as to leave the latter fluid with a well-defined outline, and much brighter than the cupel. If this clearing does not take place, the buttons are said to be frozen ; in which case the temperature must be raised, some pieces of charcoal put in the muffle, and the door closed. If they still do not clear, the heat must have been much too low, and it is best to reject them and repeat the assays.
When the buttons have cleared it is well to check the draught of the furnace, and to partly open the door of the muffle, so as to work at as low a temperature as is compatible with the continuation of the process. Too low a temperature is indicated by the freezing of the buttons and the consequent spoiling of the assays. Experience soon enables one to judge when the heat is getting too low. A commoner error is to have the heat too high : it should be remembered that that which was high enough to clear the buttons at starting is more than sufficient to keep the process going. At the finish a higher temperature is again required : therefore the door of the muffle should be closed and the furnace urged. The finish is easily recognised. The drops of litharge which in the earlier stages flow steadily from the surface of the alloy, thin off later to a luminous film. At the end this film appears in commotion, then presents a brilliant play of colours, and, with a sudden extinction, the operation is finished. The metal again glows for an instant whilst becoming solid.
If the button is a small one the cupel is withdrawn at once and placed on that square of the cupel tray which corresponds to the position it occupied in the muffle. If, however, it is fairly large precautions must be taken to prevent spirting.
Molten silver dissolves oxygen from the air and gives it off on solidifying; the escape of the gas on sudden cooling is violent and, by throwing off particles of the metal, may cause loss. This is called “ vegetation ” or “ spirting.” The silver is apparently solid when spirting takes place ; the crust breaks suddenly and some of the metal is forced out. The evil is best guarded against by slow cooling and avoiding draughts. With large buttons of silver precautions should never be omitted. One plan is to allow the cupels to cool in the muffle itself, the mouth being closed with hot charcoal. Another is to cover the cupel with another cupel previously heated to redness; in this case the silver cools between two hot cupels, and, of course, cools slowly. A third plan is to withdraw the cupel to the door of the muffle, holding it until it begins to get solid and then immediately to put it back into the hotter part of the muffle.
Silver remains after cupellation in flattened elliptical buttons, adhering but only slightly to the cupel. Its upper surface should show faint markings as if it were crystalline. The presence of platinum renders it still more crystalline, but removes the characteristic lustre and renders the metal dull and grey. Copper, if not completely removed, has a very marked effect on the appearance of the button : the metal is spread out, damping, as it were, and firmly adhering to the cupel, which latter in the neighbourhood of the metal is almost black with oxide of copper. Sometimes the silver button is globular, or even more sharply rounded on its under than on its upper surface; it is said that this is due to the presence of lead. Gold may be present even to the extent of 50 per cent, without showing any yellow colour.
The appearance of the cupel affords some useful information. The presence of cracks evidently due to shrinkage indicates a badly made cupel. If, however, they are accompanied by a peculiar unfolding of the cupel, the margin losing its distinctness, it is because of the presence of antimony. When lead is the only easily oxidisable metal present, the stained portion of cupel is yellow when cold. A greenish tint may be due to small quantities of copper or, perhaps, nickel, cobalt, or platinum. Larger quantities of copper give a greenish grey or almost black colour. A dark green and corroded cupel may be due to iron. Rings of pale-coloured scoria may be due to tin, zinc, antimony, or arsenic. When the cupel shows signs of the presence of these metals in objectionable quantity, it is well to repeat the assay and scorify so as to remove them before cupellation.
The button should be detached from the cold cupel by seizing with a pair of pliers: the under surface should be distorted by squeezing or hammering the button so as to loosen the adhering bone ash. The cleaning is easily completed by rubbing with a clean hard brush. After cleaning the buttons are best put on a tray of marked watch-glasses, and then taken to the balance and weighed. The weight of silver got needs a small correction; (1) by deducting for the amount of silver introduced by the lead or oxide of lead used in the assay; and (2) by adding for the cupellation loss.
Silver Loss in Cupellation
During the whole process of cupelling a silver lead alloy a more or less abundant fume may be observed rising from the cupel. This furnishes an evident loss of lead and a possible loss of silver; for although silver at the temperature of cupellation gives off no appreciable vapour, it is known that such fume formed on a large scale contains silver. It is, however, difficult to believe that the small amount of lead vapourised carries with it a weighable amount of silver. That it does not do so in the ordinary way of working is shown by the fact that a button of silver equal in weight to the silver lost in cupelling may be got by smelting the cupel and cupelling the resulting button of lead. The loss of silver by volatilisation is altogether inconsiderable, unless the temperature at which the operation is performed is much too high.
Another possible source of loss is the infiltration of small particles of alloy into the cupel. The cupel is necessarily porous, and particles of metal may perhaps drain into it, more especially if the bone ash is not in fine powder; but if this is the main source of loss it is hard to see why, in cupelling equal weights of silver and gold, the loss is not equal in each case. It is not easy to believe that the mere filtration of the fused alloy will effect such a change in the proportion of the metals as that which actually occurs. For example: a cupel on which an alloy consisting of 0.80 gram of silver, 0.47 gram of gold, and 25 grams of lead had been cupelled, was found to contain 7½ milligrams of silver, and rather less than half a milligram of gold. Assuming, for the sake of argument, that the gold present had filtered into the cupel in the form of small drops of alloy, it would have been accompanied by less than a milligram of silver, and the presence of the extra 6 or 7 milligrams of silver must have been due to a different cause. There can, thus, be little doubt that the cause of the greater part of the “ cupellation loss ” is a chemical one and cannot be counteracted by a mechanical contrivance. In cupellation, then, there is a loss, apart from imperfect working, inherent in the process itself; and as the amount of this loss varies under different conditions, it is necessary to study it somewhat in detail.
The following experiments are taken without selection from the work of one student. Three experiments were made for each determination, and the mean result is given. By “ range ” is meant the difference between the highest and lowest result and the percentage loss is calculated on the silver present. The silver added in the lead used has been deducted.
Effect of Varying Lead
In each experiment 0.4 gram of silver was taken and cupelled with the lead. The silver loss and “ range ” are expressed in milligrams.
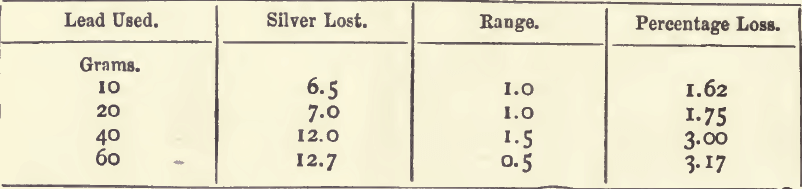
The loss increases with the lead used.
Effect of Varying Temperature.—0.4 gram of silver was cupelled with 20 grams of lead.

The difference in temperature in these experiments was much greater than would occur even with careless work.
Effect of Varying Silver
20 grams of lead were used in each cupellation.
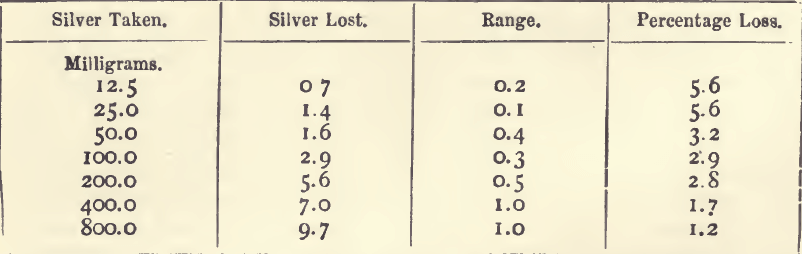
It will be seen that, although the quantity of silver lost increases with the silver present, the percentage loss is greater on the smaller buttons.
The following results are often quoted:—Cupelling 1 grain of silver with 10 grains of lead, the loss was 1.22 percent.; 10 grains of silver with 100 grains of lead, loss 1.13 per cent.; 25 grains of silver cupelled with 250 grains of lead, lost 1.07 per cent. The proportion of silver to lead was the same in the three experiments, and the largest button gave the best result. Evidently, if the quantities of lead had been the same in the three experiments (say, 250 grains in each case), the loss on the smaller quantities of silver would appear worse in the comparison.
In judging these results, it must be borne in mind that it is difficult to regulate the temperature, &c., in consecutive experiments so as to get exactly similar results, so that the range in consecutive cupellations is greater than that in a batch cupelled side by side.
Effect of Copper and Antimony.—0.1 gram of silver was cupelled with 20 grams of lead, and to one batch 0.5 gram of antimony, and to another 0.5 gram of copper was added.
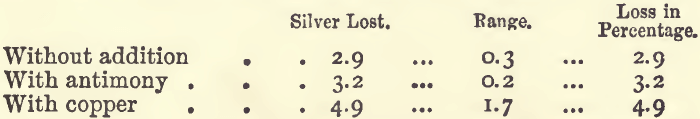
Perhaps the antimony has so small an effect because it is eliminated in the earlier part of the process, while the silver is still alloyed with, and protected by, a large proportion of lead; whilst the copper on the other hand makes its fiercest attack towards the close, when the silver is least capable of resisting it. The ill effects of copper are most strongly felt when the quantity of lead present is not sufficient to remove it: the coppery button of silver got under these conditions is very considerably less than the weight of silver originally taken.
Although the above is a fair statement of the loss attending average work, it will not do in very important and exact work to place too much reliance on the figures given, or, indeed, on any other set of figures, with the object of correcting the result of an assay. Each man must rely on his own work.
It is easy to determine what allowance must be made for the loss in cupellation by cupelling side by side with the assay piece an alloy of similar and known composition. For, if the two pieces are very nearly alike, we may justly conclude that the loss on each will be the same; and if, further, we take the average of three or four such determinations we shall get results accurate within 0.1 per cent. The method of getting such results may be best explained by one or two illustrations. This method of working is termed “ assaying by checks.”
Suppose we have an alloy of silver and lead in unknown proportions and that by cupelling two lots of 10 grams each there is got from I. 0.1226 gram of silver, and from II. 0.1229 gram. We should know from general experience that the actual quantity of silver present was from 2 to 4 milligrams more than this. To determine more exactly what the loss is, the following plan is recommended :—The two silver buttons are wrapped up each in 10 grams of lead, and cupelled side by side with two other lots of 10 grams of the original alloy. If now the two buttons I. and II. weigh 0.1202 and 0.1203, they will have suffered in this second cupellation an average loss of 2.5 milligrams. Suppose the two fresh lots of alloy gave 0.1233 and 0.1235 silver, the average loss on these would also be 2.5 milligrams. Add this loss to each result, and take the mean; which is in this case 0.1259.
If copper is present in the alloy as well as silver, it is necessary to add about the same quantity of copper to the checks as is supposed, or known, to be present in the assays. If the substance to be assayed is an alloy of silver and copper, first cupel o. 5 gram of it, with, say, 10 grams of lead, and weigh the resulting button of silver, in order to get an approximate knowledge of its composition. Suppose the button weighs 0.3935 gram. We know that this is below the truth : for the sake of round numbers take it as 0.4, and assume that the rest of the alloy (o. 1 gram) was copper. Two check pieces are then weighed out, each containing 0.4 gram silver and 0.1 gram of copper wrapped in 5 grams of lead. Of course the silver must be pure. And there is also weighed out two (or better, four) assay pieces each containing half a gram of the alloy wrapped in 5 grams of lead. The whole lot are then cupelled as nearly as possible under the same conditions. With four assay pieces, the cupels should be placed close together in two rows of three across the muffle; the two check pieces are put in the middle cupels. Suppose the buttons of silver got weighed as follows:—
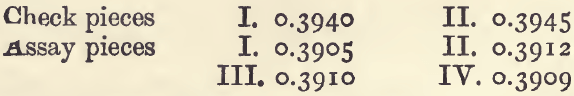
The average loss on the two check pieces is 5.7 milligrams, and the average result of the four assay pieces is 0.3909. Add the average loss to the average result, and there is got the corrected result, 0.3966. And if 0.5 gram of alloy contain 0.3966 of silver, 1000 will contain 793.2 of silver, and this is the degree of fineness.
A correction for the loss in cupellation is always made in this way when rich alloys are being assayed; and in the case of rich ores it may be done after the manner of the first of the above illustrations. There is another method of working which relies more on experiment. This is to smelt the cupel as described further on (p. 114), and to again cupel the resulting button of lead. The button of silver got in this second cupellation is added to that first obtained. It will sometimes, but not often, happen that the two buttons together will slightly exceed in weight the silver which was actually present. This is because of the retention in the buttons of a small quantity of lead. It has been stated that the proportion of lead thus retained may be as much as 1 % of the silver present; this, however, can only be under exceptional conditions. A determination of the actual silver in the buttons got in the series of cupellations quoted on pages 102, 103, gave an average percentage of 99.85, so that even with the larger buttons the effect of the retained lead would be only to increase the weight by about 1 milligram. In the method of working with checks, the retained lead has no disturbing influence.
The proportion of lead required for the cupellation of any particular alloy requires consideration. With too much lead the time occupied in the process is increased, and so is the loss of silver ; on the other hand, too little lead is of greater disadvantage than too much. From 8 to 16 parts of lead are required for each part of silver alloy, or, if gold is present, about twice as much as this must be used. For the cupellation of 1 gram of a silver copper alloy containing different percentages of copper, the following quantities of lead should be used :—

The alloy, in not too large pieces, is wrapped in the required weight of lead foil and charged into the cupel at once; or the lead may be put in first, and, when the cupellation has fairly started, the alloy may be added wrapped in tissue paper; or a portion of the lead may be first started and the alloy wrapped in the remaining lead and subsequently added. The cupellation of large quantities of alloy or of alloys which contain tin, antimony, iron, or any substance which produces a scoria, or corrodes the cupel, must be preceded by a scorification. The advantages of this are that the slag is poorer in precious metal than that found on a cupel and is more easily collected and cleaned; that larger quantities of metal can be treated, and that, even if the substance is in part infusible, or produces at the start a clinkery mass or scoria, the oxide of lead gradually accumulates, fluxes the solid matters, and produces a good final result; but if the oxide of lead by itself is not sufficient for the purpose, borax or some other flux can be easily added.
If the button of silver got is very small its weight may be estimated from its size; but it must be remembered that the weight varies as the cube of the diameter. If one button has twice the diameter of another it is eight times as heavy and so on. Scales specially constructed for measuring silver and gold buttons may be purchased; but it is much better to make the measurement with the help of a microscope provided with an eye¬piece micrometer.
If the length of the long diameter of a silver button be taken the following table will give the corresponding weight in milligrams :—
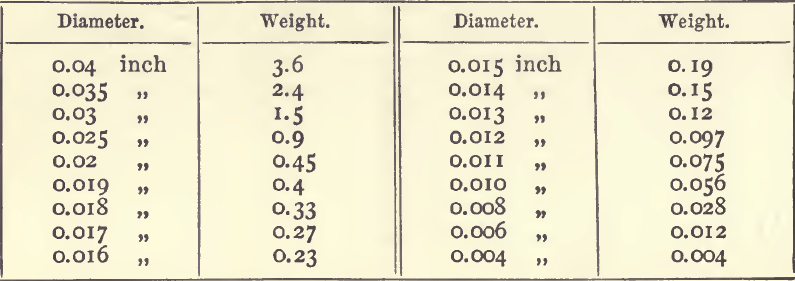
The weight of a corresponding button of gold is got by multiplying by 2.25. These figures are based on those given by Plattner, and apply only to buttons of such shape as those left after cupellation. A sphere of silver o.01 inch in diameter would weigh 0.09 milligram, and a similar sphere of gold weighs 0.167 milligram.
It is safer, however, to compare with a micrometer the diameter of the button whose weight has to be determined with that of a standard button of nearly equal size whose weight is known. The weights of the two buttons are proportional to the cubes of their diameters.
Calculation of the Results
After deducting for the silver added, and correcting for the cupellation loss, the calculation is made in the usual way; reporting as so many parts per thousand in the case of rich alloys and as so many ounces and pennyweights, or better as ounces and decimals of an ounce, in the case of poor alloys and ores.
In this last case, however, it is less fatiguing to refer to a set of tables which give, either directly or by means of simple addition, the produce corresponding to any weight obtained from certain given weights of the substance. The following table gives the produce in ounces and decimals of an ounce per ton of 2240 pounds:—
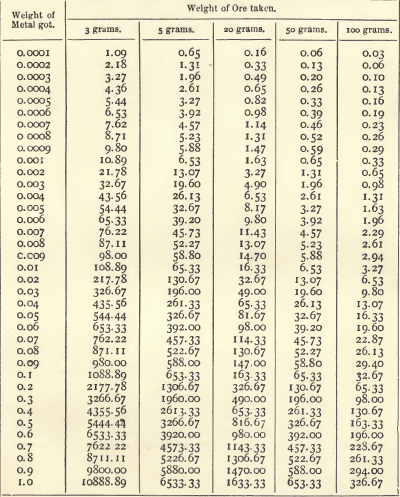
When, as in this table, the fraction of an ounce is expressed by two places of decimals, it may be reduced to pennyweights (dwts.) by dividing by 5. For example, 0.40 of an ounce is 8 dwts. The fraction of a dwt. similarly expressed may be converted into grains with sufficient exactness by dividing by 4. Thus, 1.63 ozs. equal 1 oz. 12.60 dwts., or 1 oz. 12 dwts. 15 grains. In England it is usual to report in ounces and decimals of an ounce.
The way to use the table is best shown by an example. Suppose a button of silver weighing 0.0435 gram was obtained from 20 grams of ore. Look down the 20-gram column of the table, and select the values corresponding to each figure of the weight, thus:—

Add these together. The produce is 71.05 ozs., or 71 ozs. 1 dwt. to the ton.
Or, suppose an ore is known to contain 1.24 per cent, of silver. Look down the 100-gram column, select the values, and add them together as before.
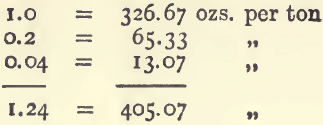
This gives 405 ozs. 1 dwt. 10 grains to the ton.
The calculation becomes more complicated when, as is frequently the case, the ore contains metallic particles. These show themselves by refusing to pass through the sieve when the ore is powdered. When they are present, a large portion, or if feasible the whole, of the sample is powdered and sifted. The weights of the sifted portion and of the “ metallics,” or prills, are taken; the sum of these weights gives that of the whole of the sample taken. It is very important that nothing be lost during the operation of powdering.
Each portion has to be assayed separately. It is usual to assay a portion of the sifted sample, say, 20 or 50 grams, and to add to the produce of this its share of the “ metallics.” This way of calculating, which is more convenient than correct, is illustrated by the following example:—

Twenty grams of the sifted portion, when assayed, gave 0.1050 gram of silver. The whole of the “ metallics ” scorified and cupelled gave 0.842 gram of silver. Since the 20 grams assayed was 1-2oth of the whole, 1-2oth part of the 0.842 gram of silver (from the metallics) must be added to its produce. We thus get 0.1471 gram (0.1050 + 0.0421).
Referring to the 20 gram column, we get—

A more legitimate method of calculation is as follows :—Calculate separately the produce of each fraction as if they were from different ores. Multiply each produce (best stated in per cents.) by the weight of the corresponding fraction. Add together the products, and divide by the weight of the whole sample. Taking the same example for illustration, we have:—
Metallics. Weight 1 gram.
1 gram of it yielded 0.842 grams of silver.
∴ Produce = 84.2 per cent.
Produce multiplied by the weight is still 84.2.
Sifted Portion. Weight 399 grams.
20 grams of it yielded o. 105 gram of silver.
∴ Produce = 0.525 per cent.
Produce multiplied by weight (0.525 x 399) is 209.475.
Add together ; and divide by 400, the weight of the whole sample
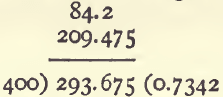
0.7342 is the total produce of the ore in per cents.
Referring to the 100-gram column in the table we find 239.84 ounces to the ton as the produce.
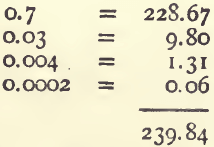
Comparing this with the result calculated by the first method — viz., 240.26, we see that that was 0.38 oz., or between 7 and 8 dwts. too high.
With ores containing “ metallics ” it is of great importance to powder the whole of the selected sample without loss during the process; and of even greater importance to well mix the sifted portion, of which the last portions, to come through the sieve are apt to be more than ordinarily rich through the grinding down of some portions of the metallic prills.
Remarks on Cupellation of Silver
Cupellation is at once the neatest and the most important of the dry methods of assaying. Its purpose is to remove easily oxidisable metals, such as lead and copper, from silver and gold, which are oxidisable with difficulty. Metals of the first class are often spoken of as base, and gold and silver as noble metals.
When lead is exposed to the action of air at a temperature a little above redness, it combines with the oxygen of the air to form litharge, an oxide of lead, which at the temperature of its formation is a liquid. Consequently, if the lead rests on a porous support, which allows the fused litharge to drain away as fast as it is formed, a fresh surface of the lead will be continually exposed to the action of the air, and the operation goes on until the whole of the lead has been removed. Silver or gold exposed to similar treatment does not oxidise, but retains its metallic condition ; so that an alloy of lead and silver similarly treated would yield its lead as oxide, which would sink into the support, while the silver would remain as a button of metal.
The porous support, which is called a cupel (fig. 5), should absorb the slag (oxide of lead, etc.) just as a sponge absorbs water, but must be sufficiently fine-grained to be impervious to the molten metal. At first sight it appears difficult to filter, as it were, a fluid slag from a fluid metal; but an ordinary filter-paper damped with oil will allow oils to run through and yet retain the water; but damped with water it will allow water to run through and retain oils. Similarly, fused slags damp and filter through a cupel, but the molten metal not damping it withdraws itself into a button, which is retained. Although, of course, if the cupel is very coarse-grained the metal may sink into the hollows.
Copper, antimony, tin, and most other metals, form powdery oxides, which are not of themselves easily fusible, and it is necessary when these are present to add some solvent or flux to render the oxide sufficiently fluid. Fortunately, oxide of lead is sufficient for the purpose ; hence, mixed oxides of copper and lead, provided the lead is present in proper proportion, form a fluid slag. In separating copper from silver or gold, advantage is taken of this fact; for, although we cannot cupel an alloy of copper and silver, it is easy to cupel an alloy of copper, silver and lead. If, however, the lead is not present in sufficient quantity, the whole of the copper will not be removed, and the button of silver, still retaining copper, will be found embedded in a coating of black oxide of copper. Copper oxidises less easily than lead does; and, consequently, the alloy which is being cupelled becomes relatively richer in copper as the operation proceeds. It is on this account that the ill-effects of the copper make themselves felt at the close of the operation, and that the oxide of copper is found accumulated around the button of silver. Tin and antimony, on the other hand, are more easily oxidised; and the tendency of their oxides to thicken the slag makes itself felt at the commencement: if the button of alloy once frees itself from the ring or crust of unfused oxide first formed, the cupellation proceeds quietly, and leaves a clean button of silver in the centre. But in either case the cupellation is imperfect, and should be repeated with a larger proportion of lead. An unfused and, consequently, unabsorbed slag tends to retain small buttons of alloy or metal, and thus cause serious loss.
There is a principle underlying many of the phenomena of dry silver assaying which the student should endeavour to understand ; and which serves to emphasise and explain some facts which without an explanation may present difficulties. If a button of melted lead be covered with a layer of slag rich in oxide of lead, and a second metal be added, this other metal distributes itself between the metal and slag in proportions which depend mainly upon the ease with which it is oxidised, and to a large extent upon the relative quantities of material present. Easily oxidisable metals such as zinc, iron, antimony and tin, will go mainly into the slag, and, if the proportion of the slag is large, very little will go into the metal. On the other hand, with metals oxidisable with difficulty, such as silver, gold, and platinum, the reverse holds true; nearly the whole of the metals will go into the lead, and very little into the slag. If, however, the slag be very rich, say in antimony, the lead will contain antimony; and, on the other hand, if the lead be very rich in silver, the slag will contain silver in appreciable quantity. Copper, which is near lead in the facility with which it is oxidised, will serve for the purpose of a detailed example. The results of actual analyses of metal and slag formed in contact with each other are shown in the following table:—
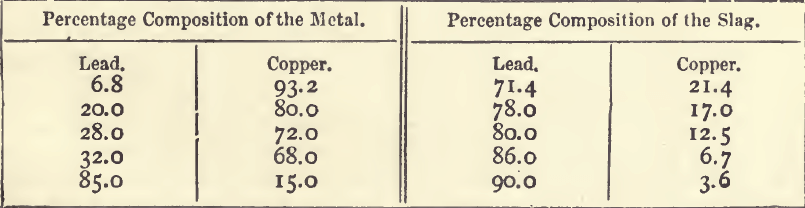
It will be seen from this table that the slag is always much richer in lead and poorer in copper than the metal with which it is in contact. The ratio of lead to copper in these five samples is:—

Assuming these figures to be correct, the following statement is approximately true. On oxidising an alloy of 10 grams of copper and 10 grams of lead, and pouring off the slag when 3 grams of lead have gone into it, there will be a loss of (owing to the slag carrying it off) about 0.2 gram of copper. On repeating the operation, the next 3 grams of lead will carry with them about 0.5 gram of copper; and on again repeating, 3 grams of lead will remove 0.8 gram of copper. Finally, the last gram of lead will carry with it 0.3 gram of copper, and there will be left a button of copper weighing 8.3 grams. The slag will have carried off altogether 1.7 gram of copper, which is 17 per cent, of the metal originally present.
With the more perfect exposure to the air, and quicker removal of the slag, which results from heating on a cupel, the loss would be heavier. Karsten got by actual experiment on cupelling copper and lead in equal proportions, a loss of 21.25 per cent.
Going back to the example: if the slag were collected and fused with a suitable reducing agent so as to convert, say, half of it into metal, that half would contain nearly the whole of the copper (such a reduction is called “ cleaning the slag ”). On re-oxidising this metal, another button of copper is formed which, added to the first, would reduce the loss from 17 per cent, to, say, 7 or 8 per cent. And it is conceivable that by a series of similar operations, almost the whole of the 10 grams of copper originally taken might be recovered. In practice the problem is (as far as the copper is concerned) not how to save, but how most easily to remove it; and since the removal of this metal is quicker from an alloy containing not too much lead, it is evident that two or three operations with small quantities of lead will be more effectual than a single treatment with a larger quantity. With those metals (tin, antimony, &c.) which pass quickly into the slag, the contrary is true; hence with these it is necessary to have enough lead present, so that the slag formed at the outset shall contain enough oxide of lead to make it fluid. As silver is so much less easily oxidised than copper, we should reasonably expect that the proportion of silver carried off in the oxide of lead would be considerably less than that of the copper indicated in the above example. Indeed, there are one or two facts which tend to encourage the hope that the operation may be conducted without any loss. If a piece of pure silver foil is exposed on a cupel to air at the usual temperature of cupellation, it undergoes very little change; it does not even fuse; it loses nothing in weight, and does not oxidise. In fact, even if oxide of silver were formed under these conditions, it could not continue to exist, for it is decomposed into silver and oxygen at a temperature considerably below redness. On the other hand, oxide of silver is not reduced to metal by heat alone when mixed with an excess of oxide of lead; while metallic silver is converted into oxide when heated with the higher oxides of lead, copper, and some other metals. That silver, and even gold (which is more difficult to oxidise than silver), may be carried off in the slag in this way, is in agreement with general experience. If 10 grams of silver are cupelled with 10 grams of lead, there will be a loss of about 50 milligrams of silver, which is in round numbers 1-3oth of the corresponding copper loss; with 10 grams of gold and 10 grams of lead, the loss will be 4 or 5 milligrams, which is about 1-12th of the corresponding silver loss.
