Table of Contents
Scrap such as insulated copper wire, small motors, automobile tires, and nonferrous concentrates from automobile shredders contains materials that become brittle at low temperatures and other materials that remain malleable. Crushing of the chilled materials to shatter the embrittled portion followed by magnetic separation, screening, and/or gravity separation was studied for recovering the individual components. Temperatures studied ranged from -30° to -195° C and were varied to suit the type of material being tested. Cryogens (chilling materials) tested included (1) dry ice with methanol for temperatures as low as -80° C and (2) liquid nitrogen to attain temperatures as low as -195° C.
Cryogenic techniques for aiding in the shredding of automobiles are presently under investigation in Liege, Belgium, by Robert George & Co. In this process, bundled automobile scrap is directly chilled by liquid nitrogen and fed to a shredder. The shredded material is magnetically separated into ferrous and nonferrous fractions. The embrittlement of the ferrous components by chilling and consequent ease of crushing supposedly offsets the cost of the procedure. In other work, a patent has been issued to J. B. Schorsch of the Union Corp., Verona, Pa. The procedure encompasses feeding insulated wire staples into a low-temperature refrigerating tank, pulverizing the embrittled insulation in crushing rolls, and screening the wire from the crushed insulation.
The research reported here was designed to extend the cryogenic embrittling technique to other types of materials to improve the economics of conventional processing. Embrittling temperatures for various materials were determined, and a system was developed for chilling materials by indirect cooling rather than by dipping the materials directly into the refrigerant. A comparison of residence times for direct versus indirect chilling is presented.
Materials Tested
Primary attention in this investigation was given to materials with appreciable metallic content such as insulated copper wires, small motors, generators, and nonferrous metal concentrates recovered from automobile shredding operations, or materials that are expensive to crush, such as automobile tires.
In the past, small motors and generators generally have been hand-dismantled, partially dismantled and leached, or directly charged into copper converters. Tires have either been shredded to separate the rubber from the beading, burned in an incinerator, or discarded in a landfill. Copper wire formerly was insulated with rubber, fabric, and paper, and was incinerated to remove the insulation. Today, copper wire insulation is principally plastic, and burning that results in the emission of noxious vapors such as chlorine is prohibited unless a scrubber is used on the incinerator to remove the noxious substance.
The small motors, generators, tires, and insulated wire used for laboratory testing were obtained from local scrap yards. The generators were removed from junked automobiles, and the small motors were removed from household appliances and automobiles. The tires were stripped from cars received for processing. The insulated copper wire treated was a typical scrap mixture of single strands and small cables. The individual wires were insulated primarily with polyethylene, polyvinyl chloride (PVC), or neoprene, although some of the wires tested were covered with silicone, Teflon, cotton, paper, or glass. In the main, the reclaimed wires were essentially pure copper, but several wires were plated with either silver or aluminum. The average weight of the wire and insulation, in percent, was 75 and 25, respectively
The nonferrous metal concentrates were obtained by processing nonmagnetic rejects from automobile shredder operations. First, the rejects were treated by air classification to remove the light material. The heavy product from this operation (about 70 percent metallics) was then treated by water classification to remove most of the remaining nonmetals. The heavy product from the water classification was 98 percent metal. After removing the iron fraction by magnetic separation, screening on 3-inch and 1-inch screens to obtain the minus 3-inch plus 1-inch fraction, and handpicking the pieces of glass from this fraction, a 100-percent-pure nonferrous metal concentrate was obtained. Recovery of nonferrous metals in this concentrate was approximately 95,5 percent.
Equipment Used
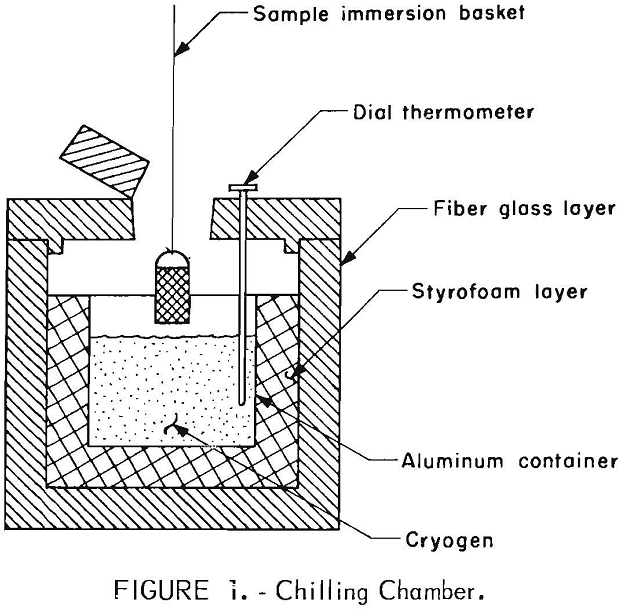
Chilling Chamber
Cryogens used in this investigation were liquid nitrogen, dry ice, and dry ice and methanol. For preliminary testing, a 5-gallon aluminum container, insulated with a 4-inch-thick inner layer of styrofoam and a 3-inch-thick outer layer of fiber glass, was used as the holding vessel (fig. 1). The materials to be chilled were placed in a copper wire basket or suspended on a piece of wire, immersed directly into the cryogen, and immediately fed to the crushing apparatus.
Crushing Apparatus
Crushing equipment used in the tests consisted of a ball mill, hammer mill, impact crusher, jaw crusher, and a set of rolls. The rolls worked most effectively with the wires, whereas the hammer mill handled the chunks of tires and shredded automobile concentrates
better than any other crushing device used. The fragmented materials were screened or water classified to produce marketable products.
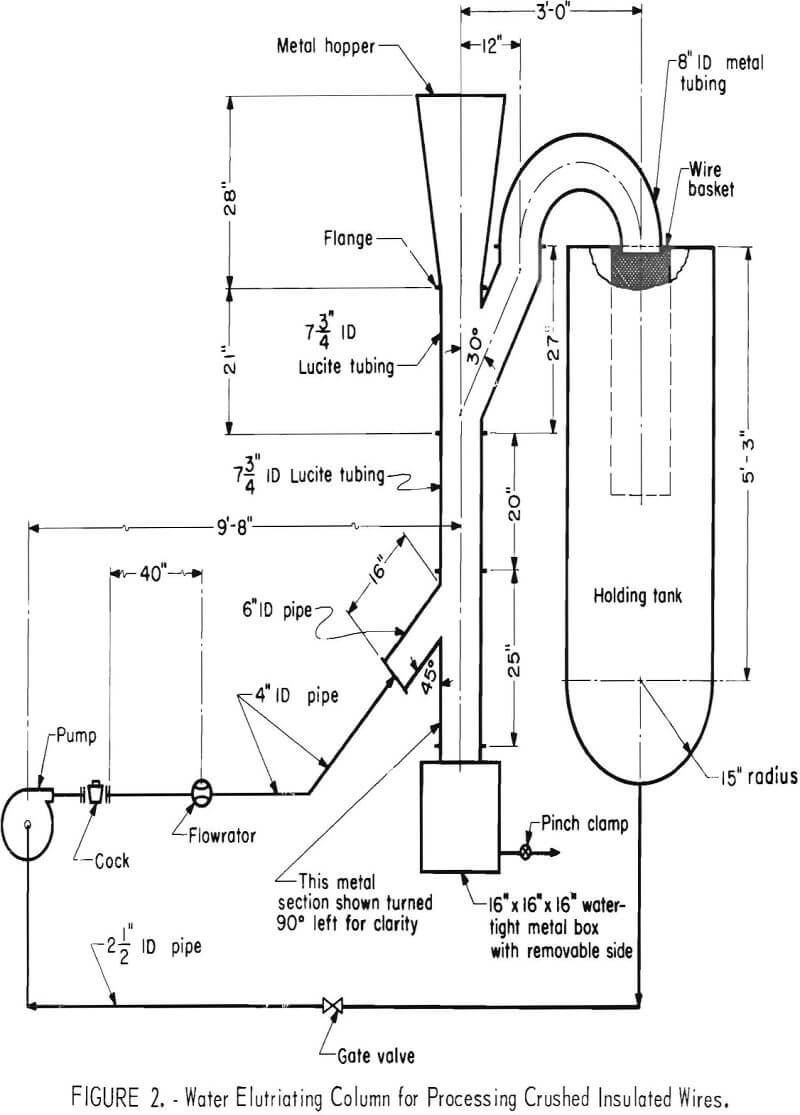
Water Elutriation and Screening
Water elutriation was the most effective method investigated for upgrading insulated wire. A schematic diagram of the elutriator used is shown in figure 2. The feed capacity of the elutriator was not established; however, it did handle without any mechanical difficulties 6-inch-long strands of wire. Size fractionation by screening appeared to be the most effective means for selectively recovering the die-cast fragments in the shredded automobile reject material.
Immersion Chilling Experimental Results
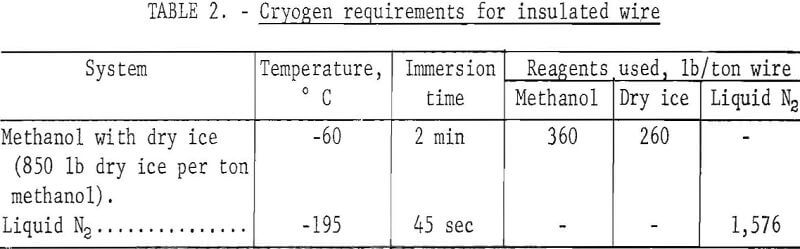
The temperatures at which various types of scrap materials become embrittled and the length of time required to induce embrittlement were first determined. Methanol with dry ice added in varying concentrations was used on materials that became brittle above -80° C and liquid nitrogen was used on materials that became brittle between -80° and -195° C. In later experiments, ½-pound samples of specific materials were processed to estimate the amount of cryogen required to treat a ton of each.
Chilling Procedures
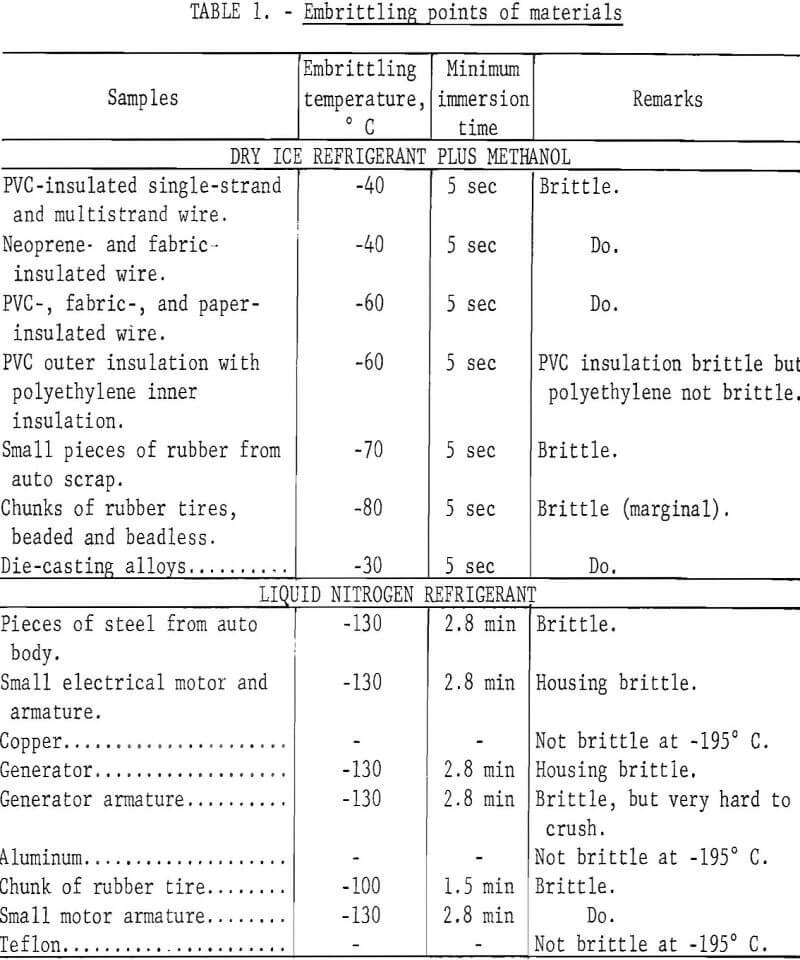
Individual samples of each of the materials tested were suspended on a piece of copper wire, immersed in the cryogen for periods ranging from 5 seconds to 10 minutes, and crushed. For the dry ice-methanol system, the temperature of the methanol was readily adjusted by varying the amount of dry ice used. Starting at -10° C, the temperature was decreased by increments of 10° until the minimum temperature of -80° C was attained. For each temperature, samples of the materials were immersed in the cryogen for a specific period of time, and immediately crushed with a hammer on an anvil. A Chromel-Alumel thermocouple in a copper well was used to monitor the temperature. Temperatures remained constant for long periods of time when chilling small samples. When used with liquid nitrogen, the thermocouple and the sample were simultaneously immersed in the liquid nitrogen and removed as soon as the desired temperature was indicated. Time required to attain the specific temperature was recorded. Results of these tests are presented in table 1.
Treatment of Insulated Wires
Several pounds of assorted 1-, 2-, and 6-inch strands of wire insulated by PVC and neoprene were processed at -60° C in methanol with dry ice and at -195° C in liquid nitrogen. The samples were placed in a ½-pound-capacity wire basket, dipped in the cryogen, and immediately dropped into a set of rolls. The crushed insulation was then separated from the wire by water elutriation. Weight of the cryogen used to process ½ pound of wire was recorded. Results of these tests were extrapolated to 1-ton samples to give the approximate cryogen requirements shown in table 2. Photographs of feed material, intermediate products, and final products are shown in figure 3.
Passing chilled wires through rolls was the most effective way of fragmenting the insulation. In some instances, the pressure from the rolls caused the fractured neoprene insulation to adhere to the wires, but the agitation in the water elutriator separated the attached insulation to produce a clean product.
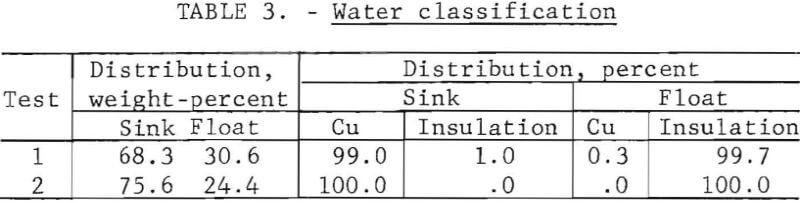
Separation of wire from insulation by water classification produced a cleaner product than that from screening or air classification. Water classification of crushed wire at a water circulation rate of 120 gallons per minute produced a sink fraction, which collected in an enclosed chamber at the bottom of the column, and an overflow fraction. Results of two water classification tests are presented in table 3; test 1 uses a methanol with dry-ice-treated product, and test 2 uses a liquid-nitrogen-treated product.

Estimate of Cryogen
Based on data presented in table 2, a rough estimate was made of the cryogen required to process insulated wires by direct chilling with liquid nitrogen or methanol and dry ice. Using approximate costs of 27 cents per gallon for methanol and $20 per ton each for dry ice and liquid nitrogen, the cost per ton to chill wire for the dry ice-methanol cryogenic system was about $17, and the liquid nitrogen cost was approximately $16. The high consumption of liquid nitrogen and methanol noted in table 2 was caused principally by wetting the surface of the wire with the respective liquid and the subsequent loss of the materials in the crushing and elutriation steps.
Treatment of Mixed Nonferrous Metal Concentrates
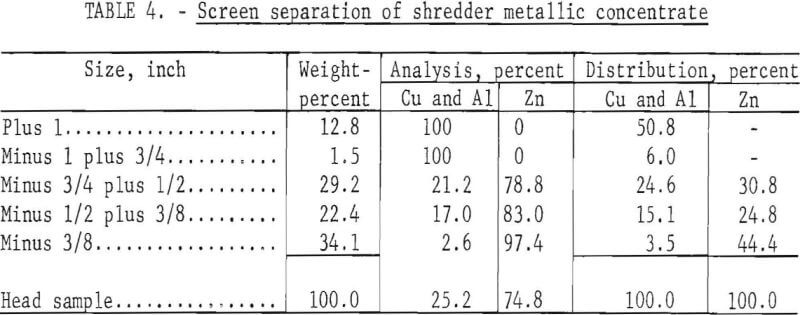
A preliminary test employing a liquid nitrogen system, crushing, and screening was run on 1,000 grams of minus 3-plus 1-inch mesh metal concentrate to separate the zinc die-casting alloys from the aluminum and copper. The concentrate for the test was obtained from shredded automobile reject material. For purposes of this report, all the values for the die-casting alloys are reported as zinc and the brass values are reported as copper although the zinc and the copper are alloyed with other metals. The composition of the shredded material after the iron and glass fractions were removed was as follows, in percent: 8.2, copper; 14.8, aluminum; 73.7, zinc; 3.3, lead. The material was chilled for 5 seconds at -30° C, crushed in a hammer mill with 1-inch grates, and screened on 1-, ¾-, ½-, and 3/8-inch screens.
The embrittled zinc die-casting alloys shattered in crushing; the aluminum and copper flattened out and were retained on the screen. The results determined by hand-sorting the screened fractions are shown in table 4; the plus ¾-inch fraction represents a recovery of 57 percent of the ductile copper and aluminum, and the minus 3/8-inch fraction represents 44 percent recovery of the zinc. Both the plus ¾- and minus 3/8-inch products were essentially free of contaminants.
Consumption of nitrogen in pounds per ton of nonferrous metal concentrates was 2,740 pounds, based on the nitrogen loss obtained in processing 1,000-gram samples. Some liquid nitrogen was trapped in the folds of the crushed materials and lost as the materials were removed from the bath.
In a supplementary test, the middling size products of minus ¾ plus ½ inch and minus ½ plus 3/8 inch shown in table 4 were refrozen, crushed, and screened on ½- and 3/8-inch screens, respectively. This test on the middling fractions showed that an additional 30 percent of the die-casting alloys could be recovered in the combined minus ½- and minus 3/8-inch fractions as a 96-percent-zinc die-cast product with a loss of only 4 percent of copper plus aluminum. A composite of the entire test showed a recovery of 57 percent of the copper and aluminum in a 100-percent product; a recovery of about 74 percent of the zinc die-casting alloys in a 97-percent product; and a secondary middling composed of 30 percent copper and aluminum and 70 percent die-casting alloys requiring additional treatment.
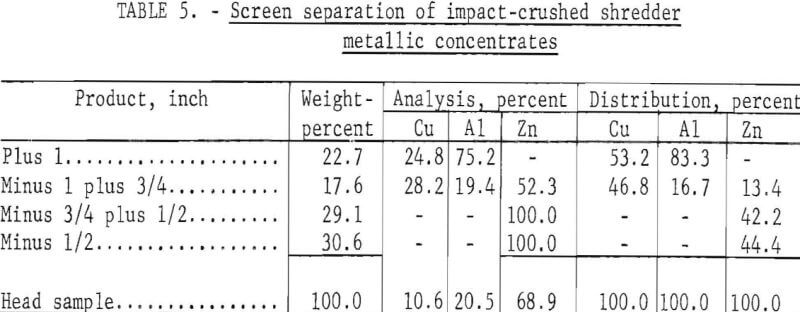
Two additional cryogenic tests were conducted on mixed nonferrous metal concentrates to determine the effectiveness of different crushing systems. In both tests a chilling temperature of -72° C and a time of 1 minute were used. Differences in results obtained were attributed to the type of crusher employed. The previous test results reported were obtained using a hammer mill with 1-inch grates. These results were compared with that of the second test, which used a locally constructed impact crusher as a primary crushing device and a jaw crusher with a ¾-inch opening for secondary crushing of the middling particles. The impact crusher consisted of a shell with hardened steel walls surrounding a vertical rotor topped by a horizontal disk with attached upright arms. The metallic feed particles were fed through a central feed chute onto the horizontal disk turning at 2,000 revolutions per minute and were flung by the arms against the surrounding walls to cause breakage. Table 5 shows the results of freezing minus 3-plus 1-inch material for the standard 1 minute at -12° C, shattering in the impact crusher, and screening in 1-, ¾-, and ½-inch screens.
The minus 1- plus ¾-inch product from the aforementioned test was rechilled to -72° C for 1 minute, recrushed in a jaw crusher of ¾-inch opening, and then screened on ¾- and ½-inch screens. Results are shown in table 6.
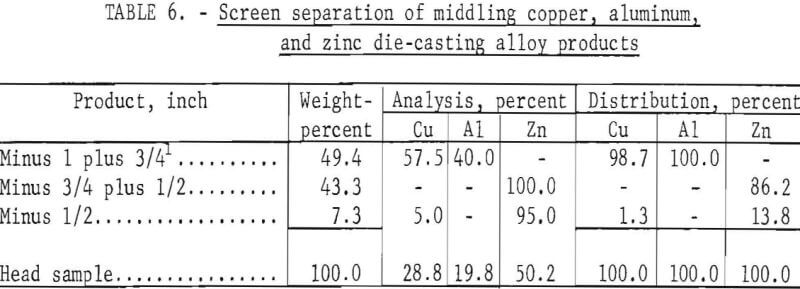
By compositing like products obtained by preliminary and secondary crushing and screening, the following products can be obtained: (1) A plus ¾-inch clean mixed copper-plus-aluminum product at an overall recovery of 99.4 percent, and (2) a minus ¾-inch product representing a 100-percent recovery of the zinc die-casting alloys contaminated by about 0.5 percent copper.
The crushing device used in the third test was the same hammer mill used for the initial test in this series but with the grate removed so that the material dropped free after being impacted by the hammer. As in previous tests, the starting material was minus 3- plus 1-inch mixed metals that were cooled to -72° C for 1 minute prior to crushing. Results obtained from a single pass through the grateless hammer mill are given in table 7; figure 4 shows the feed material A, and products B, C, D, and E, which represent the plus 1-, ¾- , ½-, and minus ½-inch fractions, respectively.

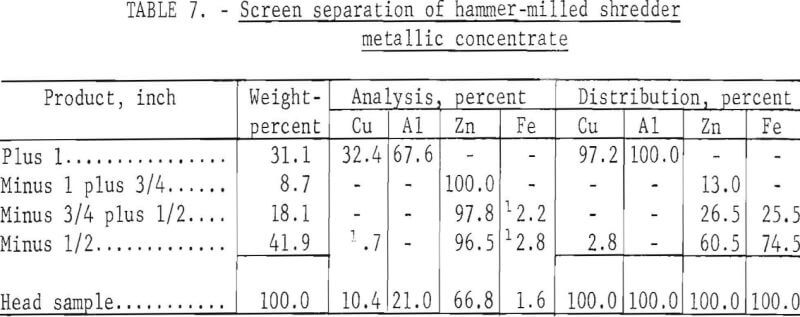
Note that excellent liberation of the metals was obtained in this test by a single pass through the grateless hammer mill. Recrushing of a middling fraction was not necessary. Compositing the sample in this test indicates that 97.2 and 100.0 percent of the copper and aluminum, respectively, are recovered in the plus 1-inch product, and 100 percent of the zinc is recovered in a minus 1-inch product of over 97-percent die-cast purity.
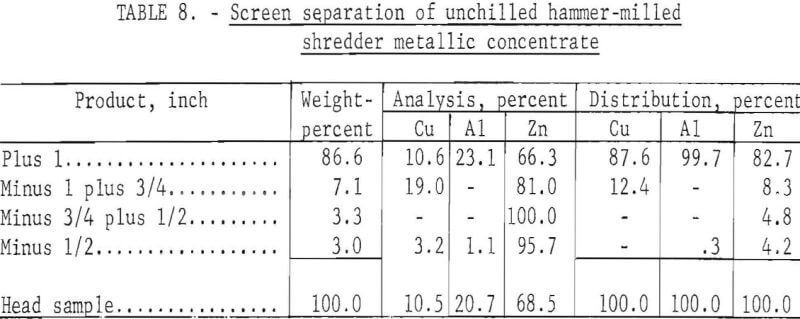
To demonstrate the effectiveness of the chilling-crushing technique, an unchilled mixture of minus 3- plus 1-inch mixed metal was crushed in a single pass through the grateless hammer mill. The results given in table 8 clearly demonstrate that chilling of the metal concentrate prior to crushing enhances the fragmentation of the zinc die-casting alloys.
Chilling of Miscellaneous Materials
Preliminary investigations were made to determine the feasibility of using the cryogenic technique for freeing the component materials in generators, small electrical motors, and rubber tires. The procedure for the generators and motors envisaged embrittling the steel, cast iron, and cast aluminum to free these materials from the contained copper. Work on the rubber tires envisaged embrittling the rubber and fabric to free them from the steel beads. Liquid nitrogen was used as the chilling medium for all tests involving these materials.
Preliminary tests were made on used passenger car tires containing the following, in percent: Approximately 85, rubber; 11, fabric; 4, steel beads. These tests showed that chilling of rubber tire sections for about 2 minutes at -100° C followed by shattering in a grated hammer mill, magnetic separation, and screening of the crushed material produced excellent separations of the steel beads, rubber, and fabric. All of the steel beads were recovered by magnetic separation. The coarser fabric material, which contained about 10 percent by weight of attached rubber, was separated from the bulk of the finer sized rubber by screening on a ½-inch screen. The minus ½-inch rubber product was essentially clean and represented over 98 percent recovery of the original rubber.
Chilling of generators, starters, and small motors followed by hand-tool crushing of the materials was not effective for metal separation. Although chilling and embrittling of the encased armature was obtained when the entire generator or starter was immersed in cryogen, crushing of the armature did not liberate the iron core from the surrounding copper wire coil. Good separations were not obtained even with a dual chilling process, in which chilling of the outer case was followed by crushing for removal and chilling of the inner armature. Hand-tool crushing of the armature was ineffective even when the armature was chilled to 196° C. Crushing chilled cases with a sledge hammer took just about as much time as did crushing unchilled cases.
Indirect Chilling
When feed material was immersed directly into liquid nitrogen or liquid methanol, appreciable cryogen losses occurred owing to surface wetting or entrapment. The loss of cryogen or heat-transmitting fluid was limited to that from wetting of the surface when treating insulated copper wire but was appreciably greater when treating mixed nonferrous metal shredder concentrates. The folds in the metals, formed during the original shredding process, apparently entrapped appreciable quantities of cryogen, which was lost during the subsequent crushing operations. To overcome these losses, consideration was given to designing a cryogenic chamber that could chill materials by indirect conduction and convection cooling.
A prototype cryogenic chamber was designed and constructed to determine the residence time required to achieve embrittlement by indirect cooling. The device basically consisted of three concentric aluminum cylinders. The central cylinder was hollow and open at both ends to serve as a holding tube into which the material to be chilled was fed. The second cylinder was closed except for an outlet port and an inlet port for feeding of the cryogen. The space between the outer cylinder and the second cylinder was filled with polyurethane foam for insulation. The material to be chilled was fed into the central cylinder by a vibrating feeder and was discharged from the bottom by a similar vibrating feeder after a suitable residence time at the chilling temperature. A schematic diagram of the indirect cooling system is shown in figure 5.
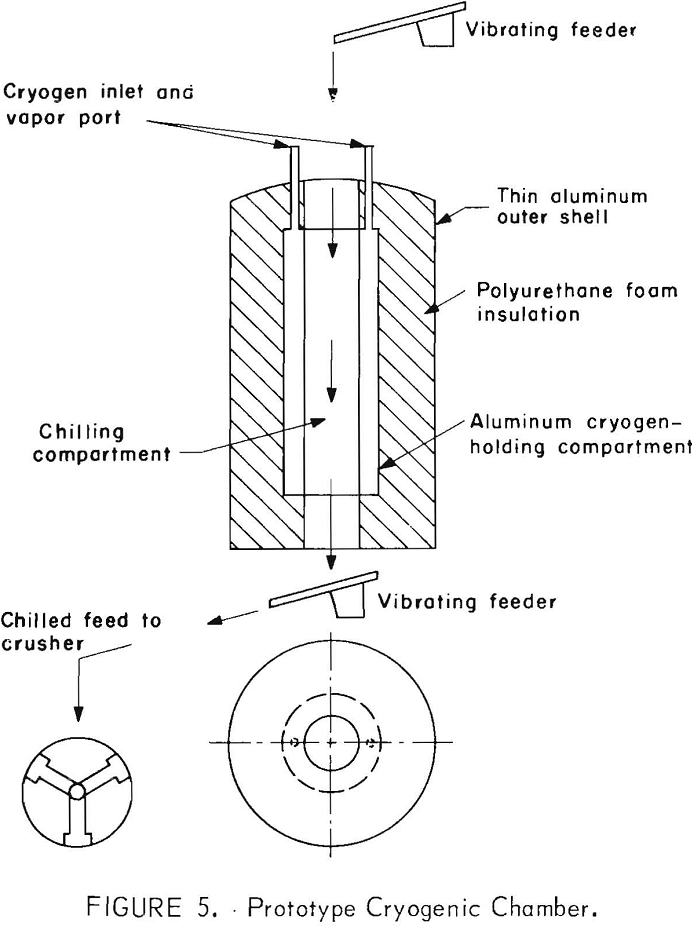
Laboratory testing of the apparatus was made using liquid nitrogen, dry ice, and methanol and dry ice as cryogens. Preliminary testing was done to obtain a comparison of the residence time required to attain a specific temperature both by direct and indirect chilling. The comparison was achieved by immersing a dial thermometer within a 1/8-inch-diameter copper mill directly into the cryogen or by suspending the thermometer in the center of 3- and 6-inch-diameter feed cylinders of the indirect system and noting the respective times required to achieve the desired temperature. Temperatures could be lowered to -185° C using liquid nitrogen but were limited to a low of -70° C using dry ice systems. Table 9 illustrates the increase in time required by indirect chilling to various temperatures compared with that of direct immersion.
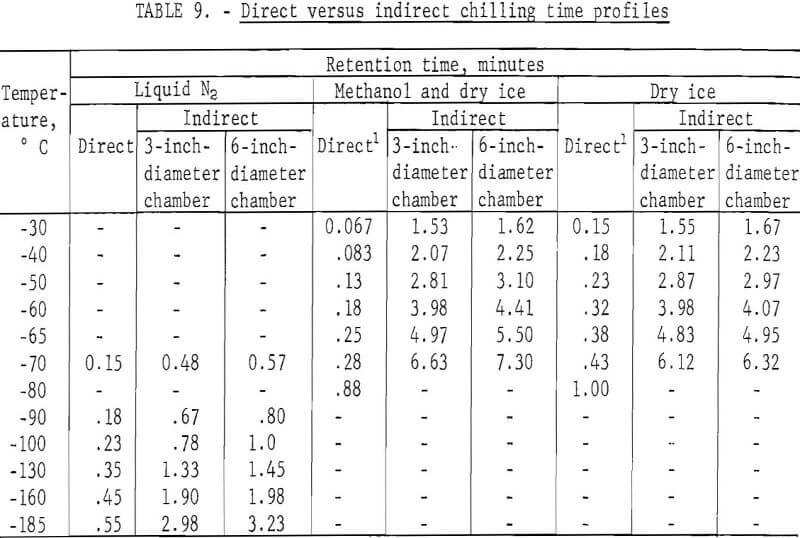
The results indicate that the increase in residence time required for indirect chilling is not excessive even in a cylinder with a diameter of 6 inches. Based on these favorable findings, a system was designed and constructed to test the effectiveness of chilling scrap samples indirectly. A liquid CO2-dry ice system was chosen over the cryogenic systems shown in table 9 for the following reasons: (1) The temperatures attained by this system are low enough to embrittle both zinc die-casting alloys and insulated copper wire; (2) liquid CO2-dry ice can be readily recycled in a closed system; (3) a liquid CO2-dry ice system is less expensive than a liquid nitrogen system.
The unit comprises a cryogenic system in which the CO2 vapors emitted by the cryogenic chamber would be recycled through a compressor to produce liquid CO2. Cooling of the scrap is obtained by evaporation of the CO2, which is recycled to the compressor. Testing of the indirect cooling system is continuing on insulated copper wires and nonferrous metal concentrates.
Conclusions
- Preliminary testing of the cryogenic procedure using liquid nitrogen, dry ice, or dry ice and methanol as cryogens indicates that the system is suitable for processing relatively high-value materials such as mixed nonferrous metal concentrates and insulated copper wires. Excellent separation of a mixture of copper and aluminum from zinc die-casting alloys and of wire from insulation were obtained when using cryogenics.
- Cryogenic processing is beneficial in reclaiming steel and rubber from scrap tires but does not appear to be beneficial in crushing and sorting metals contained in generators, starters, and small motors.
- Preliminary laboratory testing indicates that a sufficiently low temperature can be obtained by indirect chilling to permit use of a liquid CO2-dry ice system on insulated wire and mixed nonferrous starting materials.
