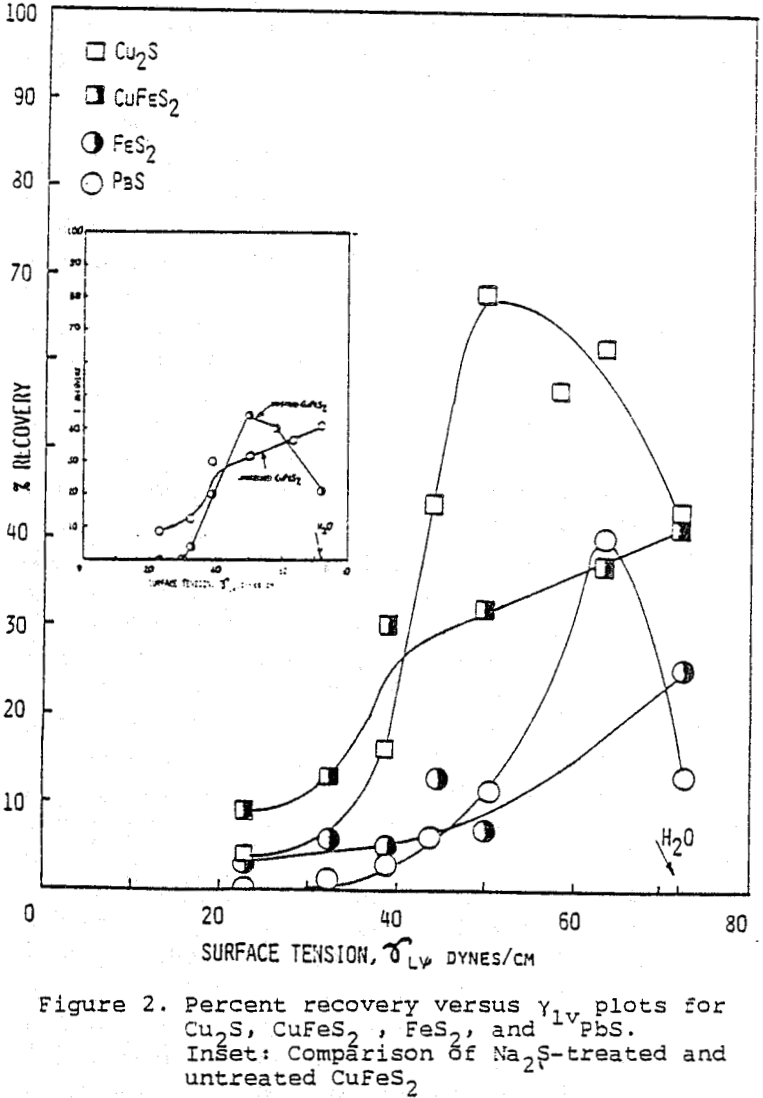
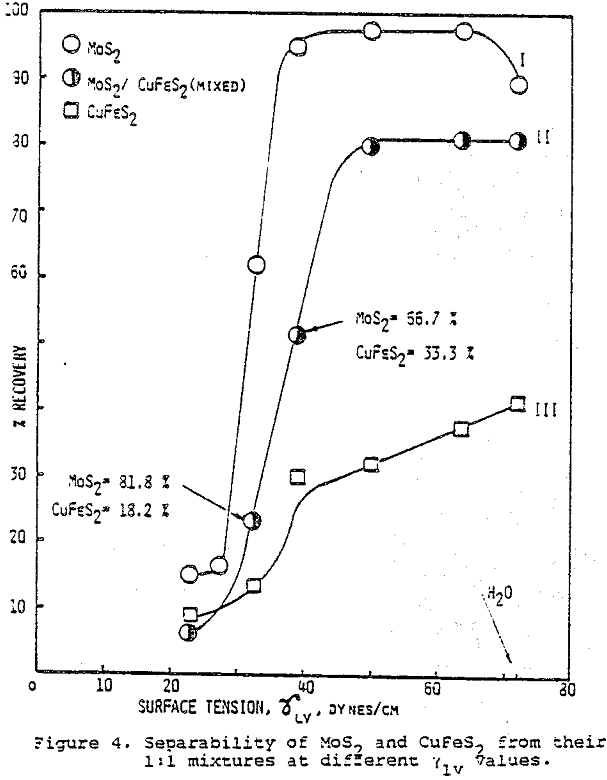
The critical surface tension of wetting (vc) of Cu2S, FeS2, PbS, and M0S2 were determined by plotting flotation recovery versus γLV which was arranged by methanol, and extrapolating to % R = 0.
γc values ranged from 26 dyne cm-¹ for M0S2 to 49 dyne cm-¹ for PbS and elemental sulfur gave a value of 31.5 dyne cm-¹.
It has, on the other hand, been customary to correlate contact angle (θ) with flotation data, although it is recognized that surface inhomogeneities and related contact angle hysteresis limit the interpretations pertaining to solid surface structures purely based on contact angle measurements. It is nonethless true that θ=0, when the liquid is water, corresponds to a hydrophilic solid.
Molybdenite (M0S2), pyrite (FeS2), chalcopyrite (CuFeS2), chalcocite (Cu2S), and galena (PbS) were hand-sorted from massive samples and ground by mortar and pestle, and the -100 mesh fraction used in experiments.
Curves obtained by the same method for the minerals Cu2S, PbS, CuFeS2, and FeS2 are given in Figure 2, where it is seen that for Cu2S and PbS, the extrapolation to % R = 0 produces γC value of 36 and 49 dynes/cm, respectively. FeS2 and CuFeS2 on the other hand, give curves which do not permit such an extrapolation to obtain a γC value with certainty.
The mechanism of such a phenomenon is not fully elucidated although the presence of elemental sulfur has been considered as the possible source of hydrophobicity.
The γc values of sulfide minerals treated by Na2S, become identical to that of elemental sulfur; and therefore, from the conclusion quoted in relation to SiO2/MoS2, it should follow that the activation of these minerals by Na2S ought to be due to elemental sulfur at their surfaces.


Dynamic Surface Tensiometry & Froth Flotation
Surface tension and its control are important in the concentration of ores by froth flotation, both in terms of producing the proper bubble size distribution and to render the ore surface of interest hydrophobic such that adhesion to a bubble will take place. The influence a substance has on surface tension has traditionally been measured using static, equilibrium techniques.
The apparatus is based on a refined bubble pressure method, with nitrogen gas passing through an orifice of known diameter which has been submerged into a solution at a predetermined depth. A unique gas feed arrangement with appropriate electronics measures and records the maximum pressure needed to generate bubbles in the time span of 1 – 10 bubbles/second or more. Hydrostatic pressure is subtracted from the maxi-mum pressure required to produce bubbles based on the density and depth of the fluid used. Deionized water is generally run as a reference to quickly calibrate the apparatus.
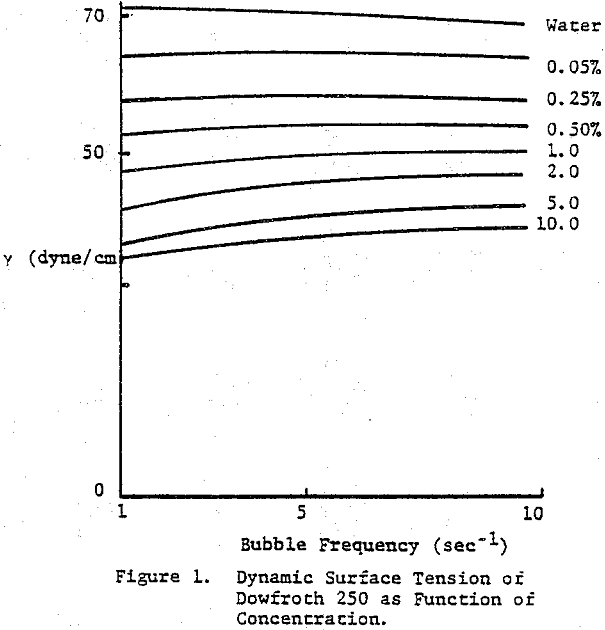
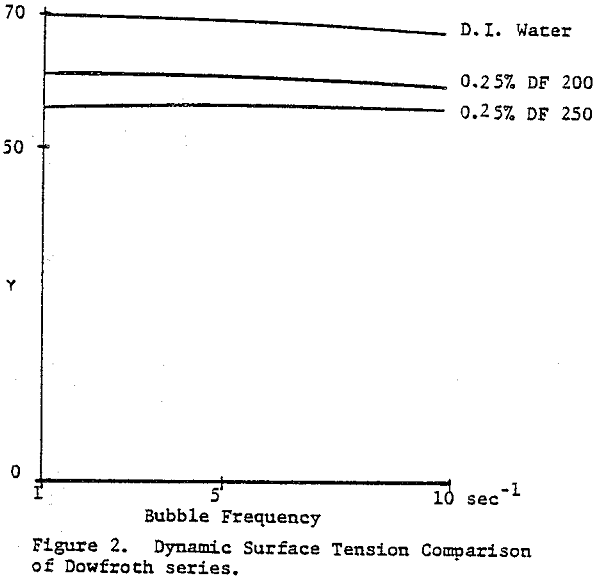
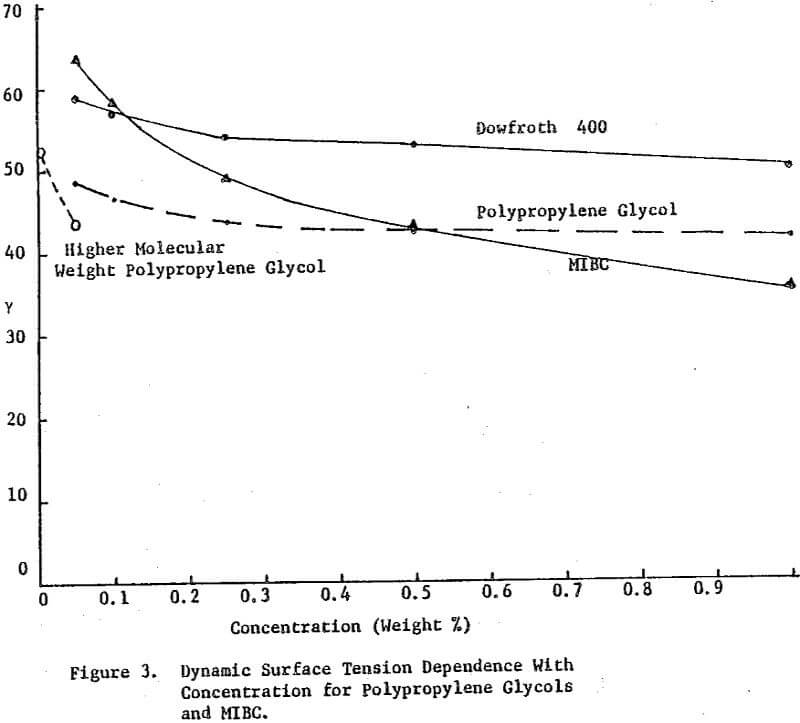
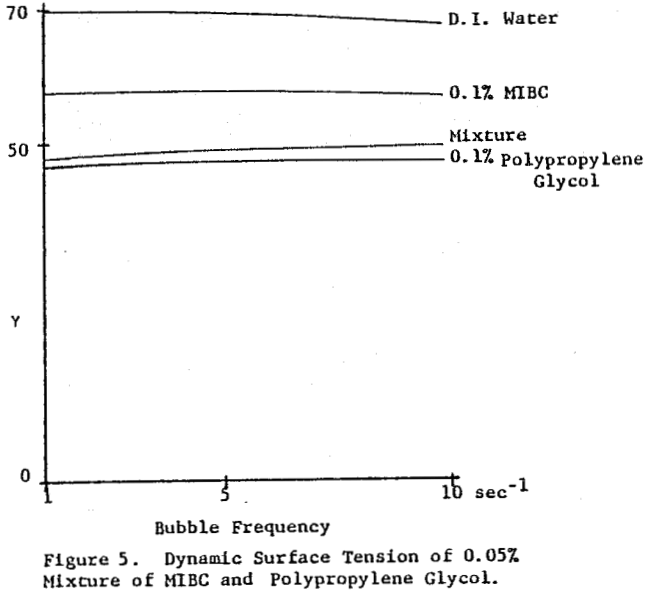
The dynamic surface tension of Dowfroth 250, a polypropylene glycol methyl ether, as a function of concentration in water was studied. The surface tension is not a function of the rate of bubble formation in this concentration range. Data for higher molecular weight versions of these polypropylene glycol methyl ethers confirms the increased surface activity as the molecular weight increases. Alcoholic frothers, such as methylisobutyl carbinol (MIBC), also exhibit a slowly decreasing surface tension in water with increasing concentration, but with a stronger concentration dependence than glycol frothers.
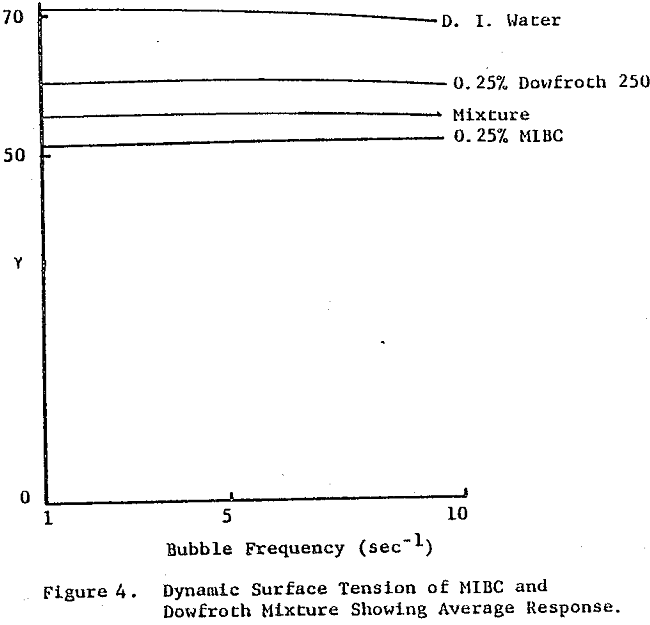
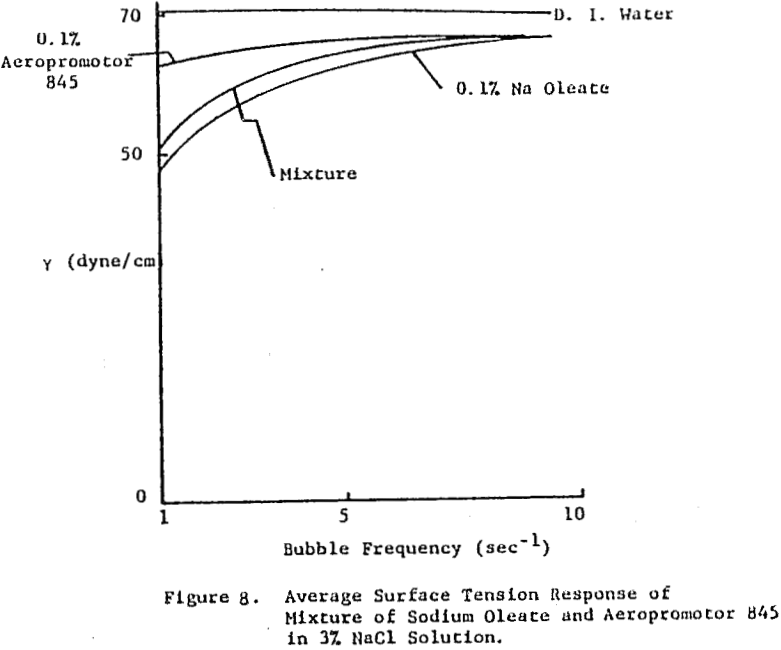
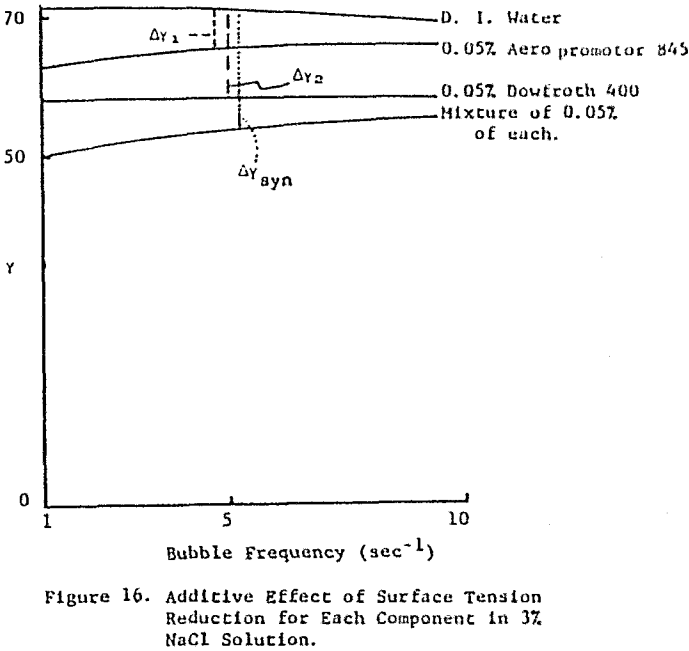
The frother blend of MIBC and Dowfroth 250 is presented in Figure 4. An average reduction in surface tension results. Similar behavior is noted for MIBC and Dowfroth 400, a straight polypropylene glycol. Some a typical systems can be found that deviate from the average surface tension reduction.
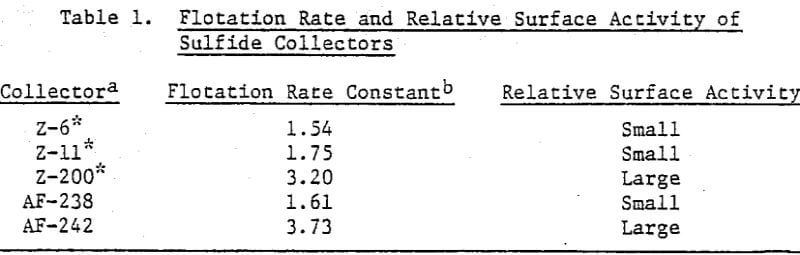
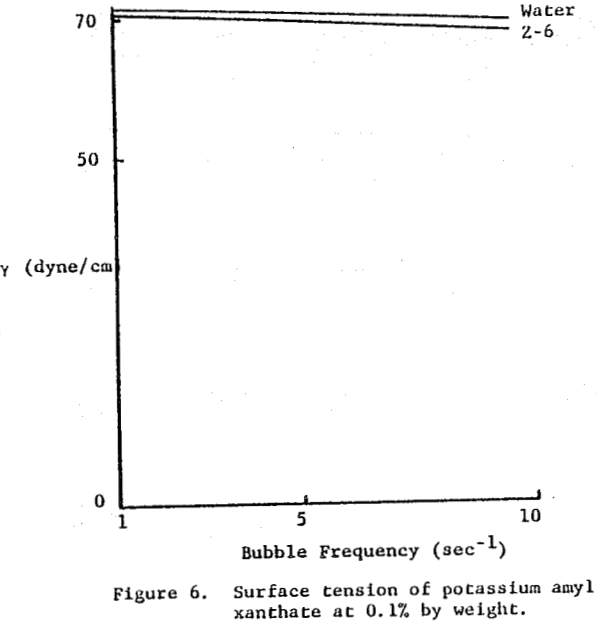
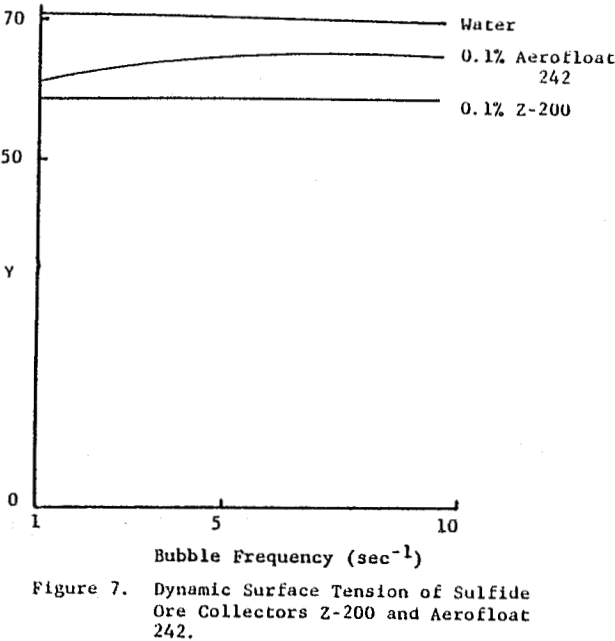
Xanthate salts used commercially in sulfide mineral flotation showed minimal surface activity towards the air-water interface. Figure 6 illustrates the surface tension response of Z-6 (potassium amyl xanthate). Similar results were seen for Z-11 (sodium isopropyl xanthate) and Z-14 (sodium isobutyl xanthate). Aerofloat-238, sodium di-secondary butyl dithiophosphate, behaved similarly.
At more rapid rates of bubble formation, insufficient surfactant molecules reach the surface to lower the surface tension to its equilibrium value. It is obvious that collectors containing alkyl groups in excess of eight carbon atoms will influence the bubble size distribution to an extent depending on the rate of bubble formation.
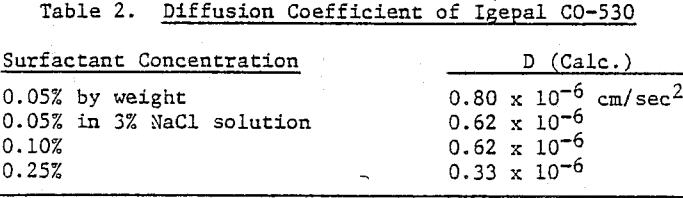
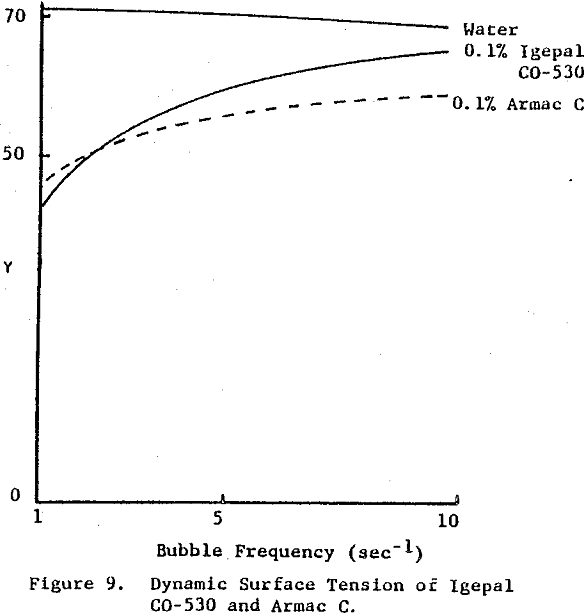
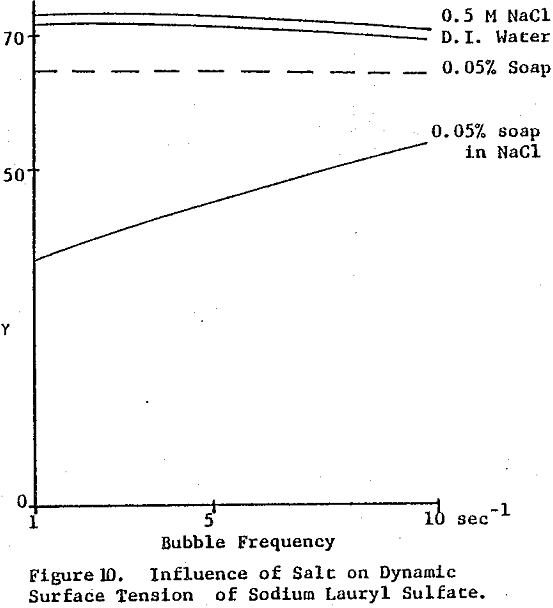
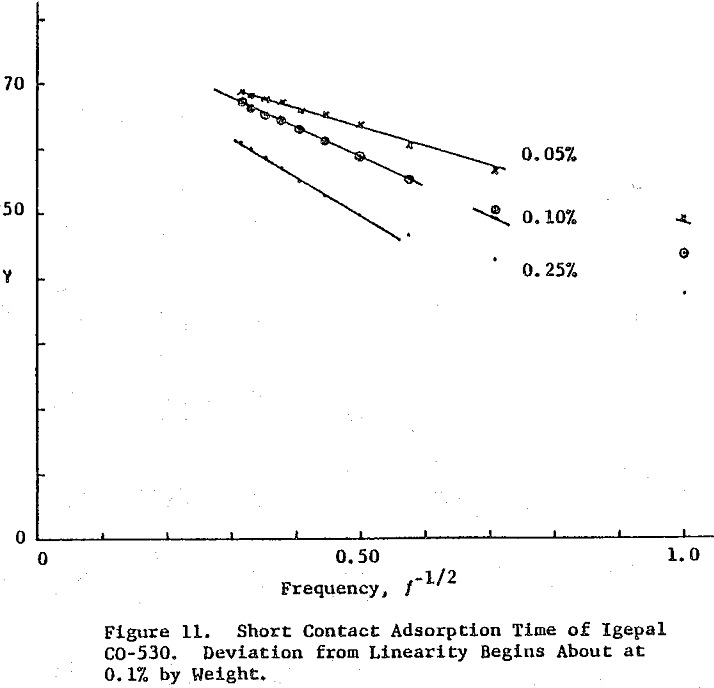
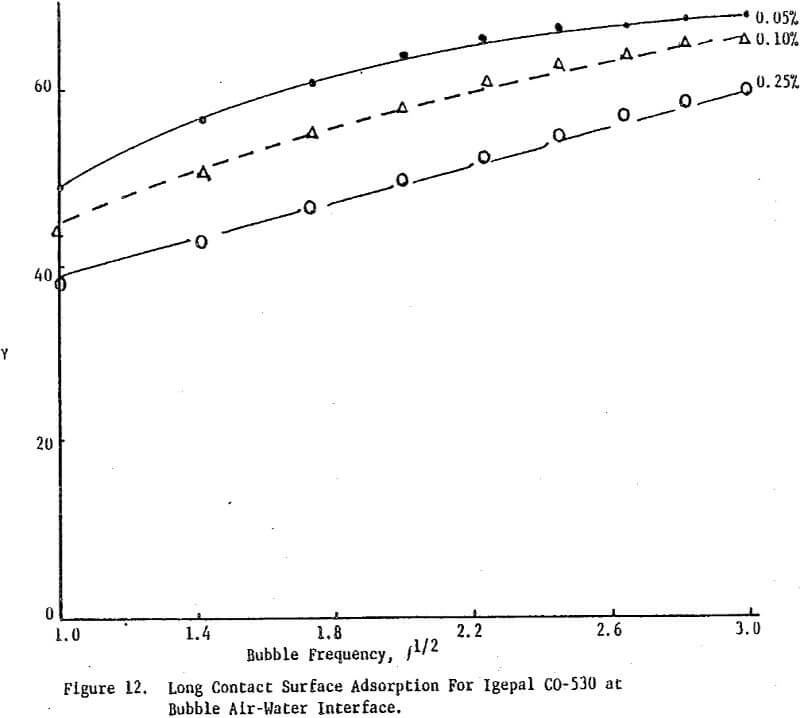
Surface tension is plotted against the reciprocal square root of bubble frequency. Deviation from linearity begins for Igepal concentrations of 0.1% by weight and above. It is noted that the diffusion coefficient is concentration dependent and also a function of salt presence. This suggests that bulk diffusion of surfactant molecules is not the sole contributor to frequency dependent surface tensions.
An example of a frother-surfactant system where the surfactant displays diffusion-limited behavior is provided by the combination of MIBC and Igepal CO-530. With their concentrations chosen such that their surface tensions cross at some bubble frequency of formation, the mixture exhibits the dynamic surface tension of the most surface active species at high bubble rates (MIBC) and a surface tension approaching that of Igepal CO-530 at low bubble frequencies. At the crossover point the combined effect of the mixture is a lower surface tension than for either component alone.
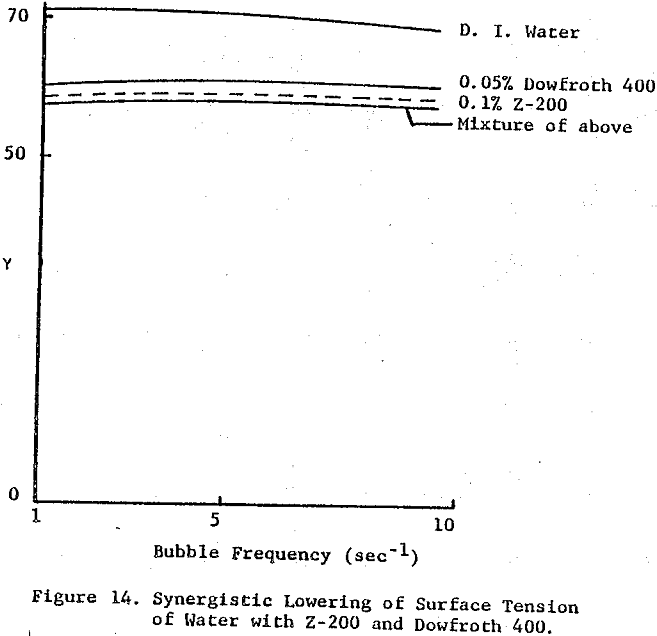
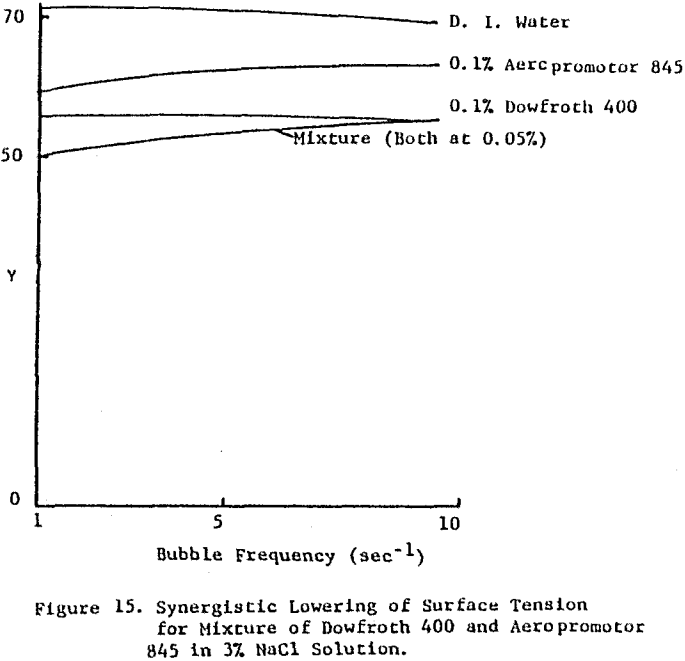
Though not proven from this work, when an enhanced rate of flotation is seen for these combinations of flotation chemicals, the effect may be due not so much from the ability of the collector to adsorb onto the surface of the bubble and be transferred to the ore surface as it is the ability of the bubble to accommodate each – surface active species independently, in effect reducing the induction time needed for permanent attachment of particle to bubble to take place.