Table of Contents
Experimental studies on the flotation of silicate minerals go back at least thirty or forty years (Bull, 1929; Bull, Ellefson, and Tylor, 1934; Fuerstenau, 1962; and Gaudin, Glover, Hansen, and Orr, 1928). These investigations for the most part have been empirical and have failed to give detailed information on the nature of the silicate-solution interface. Also, very little has been reported about the sorptive properties of silicate minerals in the presence of various collector systems.
Deju and Bhappu have made a theoretical and experimental study of the sorptive properties of representative silicate minerals in an aqueous solution.
The present study extends the previous work to include the surface phenomena occurring in a silicate-sulfonate system and in a silicate-amine system. Efforts were made to correlate the surface sorption in the presence of a sulfonate or an amine to the surface sorption in the presence of only deionized water. The term sorption rather than adsorption is used because both ion exchange and physical adsorption play important roles in the surface phenomena occurring in solid-liquid systems.
Experiments were also conducted varying the collector concentration to examine its effect both on the total surface sorption and the rate of sorption. These experimental results are compared to a theoretical model based on a Langmuir-type sorption isotherm.
Finally, the behavior of some silicate minerals in flotation was predicted from these sorption studies and an explanation for the flotation of silicate minerals postulated on the basis both of theoretical predictions and experimental results.
The authors sincerely express their appreciation to Mr. Alvin J. Thompson, director of the New Mexico Bureau of Mines and Mineral Resources, for his continued support and encouragement throughout the project and for permission to present this report. Appreciation is also extended to Drs. Dexter H. Reynolds and Geoffrey Purcell for their critical review of the paper and to Miss Teri Ray for editing the manuscript. Mr. John Bonnet, Mrs. Lynn Brandvold, Mr. Walter Fisher, and Mr. Young H. Paik helped greatly in the experimental part of the project. Mr. Lee Bessey and Mr. William Arnold of the Bureau staff did the drafting. Mrs. Lois Devlin, Mrs. Wilma Nicholson, Mrs. Mildred Wagner, and Miss Kay Williams assisted in typing the manuscript.
A Theoretical Model of Sorption Rates
In a previous paper (Deju and Bhappu, 1966), a theoretical model was developed to describe the behavior of silicate minerals when immersed in water. This model portrayed the sorption phenomena from a completely chemical standpoint.
The effect of electrostatic forces was explained and extended to igneous rocks in another paper (Deju, Summers, and Bhappu, 1966) in which expressions were derived that allow us to relate electrostatic potentials to the degree of sorption of the rocks.
These models will now be extended to include a discussion of rates of sorption.
We begin by defining the sorption of ions from the liquid to the surface of the solid by the equation
D=Ae -Bt…………………………………………………………..(1)
where D is the degree of sorption at time t, and A and B are constants. Also, let ΔpH be an index of the total sorption of H+ ions.
Then, the total change in pH after time t has elapsed will be
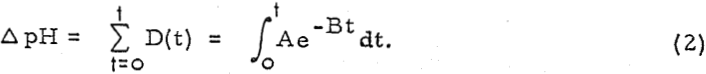
Thus, it follows that
![]()
where Co = A/B and Cl = B.
Using equation (3), the change of pH after a time t has elapsed can be determined. The constants Co and Cl must be experimentally determined. Co is readily determined since when t → ∞

Cl can be determined by differentiating equation (3).
Equation (3) will hold for any liquid phase. However, in comparing sorption isotherms obtained by using equation (3) for different liquids, the effect of ionic concentration in the liquids must be considered. This is of great importance where a reaction in deionized water is compared to a reaction in the presence of collector ions. In this instance, the presence of collector ions may make the values of Co and Cl different from those in the water.
Sorption and Sorption Rate in a Sulfonate System
A series of experiments was performed using silicate minerals to examine experimentally the effect of the sulfonate ion on sorption; that is, to examine the relation between sulfonate concentration and the constants Co and Cl. The sorption apparatus used was described in a previous paper (Deju and Bhappu, 1965a).
Materials
Sulfonates
Two sulfonates were used in the experiments. The first was a sodium sulfonate of alkyl benzene manufactured by the Monsanto Chemical Company. The hydrophilic portion of this sulfonate is SO3Na-. The average length of the carbon chain is 15. This sulfonate has a molecular weight of 390.
The other sulfonate was Santomerse 85-b, also produced by Monsanto. Santomerse 85-b is a biodegradable anionic surfactant; chemically, it is the sodium salt of a linear alkyl-aryl sulfonate. The average length of the carbon chain is 11.4.
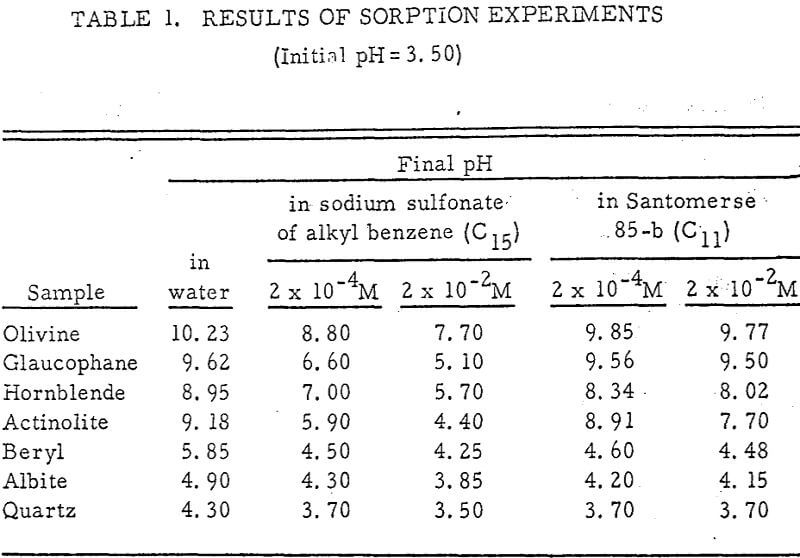
Solutions of these sulfonates of concentrations 2 x 10 -4 M and 2 x 10-² M were prepared. Hydrochloric acid was used to adjust the pH to 3. 50 for the 100-ml amounts used.
Minerals
The following minerals were used in the experiments:

The minerals were dry-crushed in a ceramic ball mill and sieved to obtain a -100 +200-mesh sample. The sized samples were then run through the electromagnetic separator to remove any magnetic particles.
Procedure
Ten-gram samples of the minerals were placed in 100 mls of freshly deionized water or of sulfonate solution which was previously adjusted to the desired pH. The polypropylene beaker containing the sample and the water was placed under a bell jar from which the air was displaced at once by nitrogen gas. The sample and solution were stirred gently but continuously during the entire experiment, the stirring speed being kept constant for all runs. The temperature was approximately 25° C. Changes in pH were followed using a Corning Model 12 pH meter and were recorded on a Heathkit Model EU-20.
Results
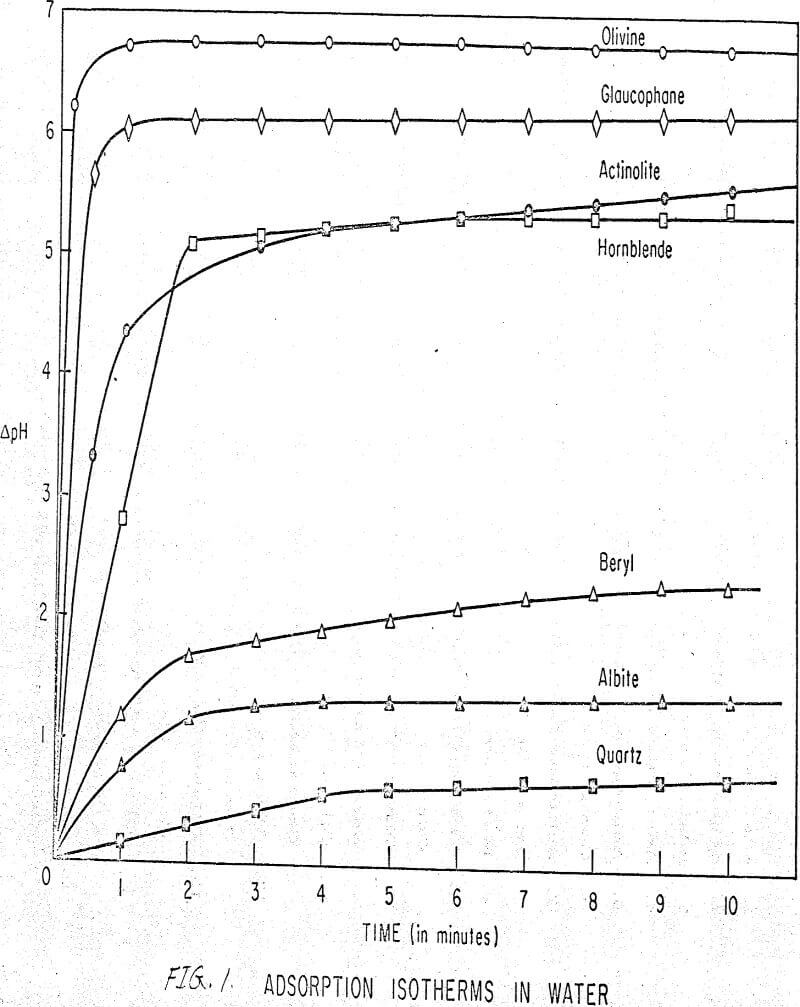
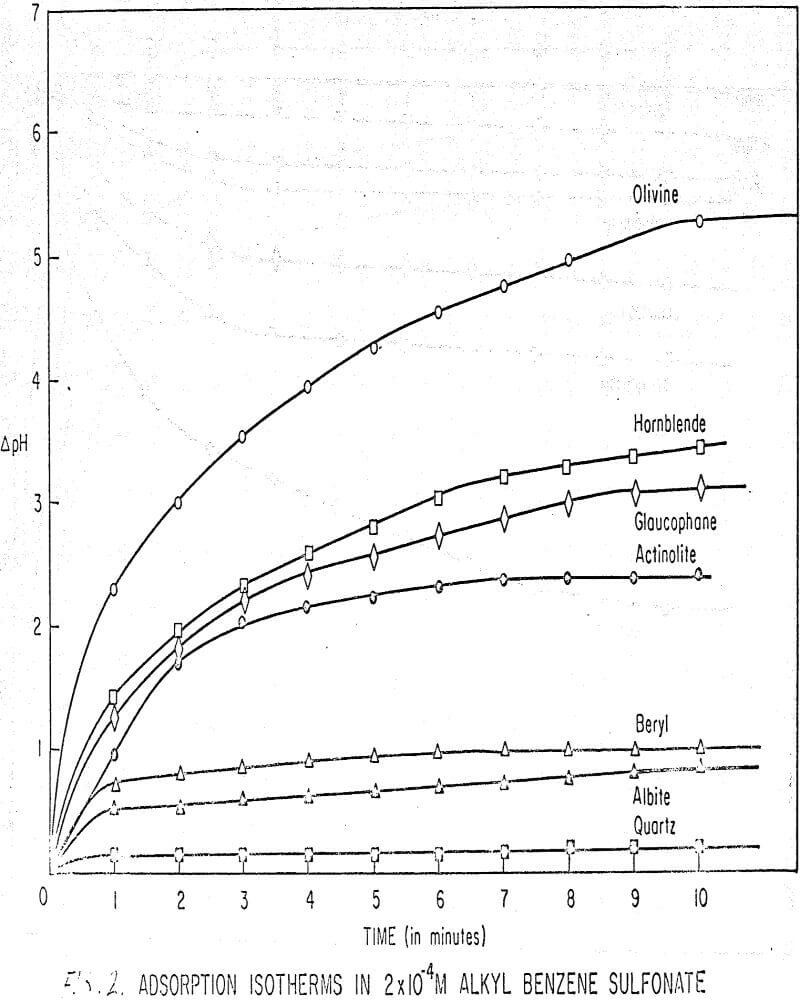
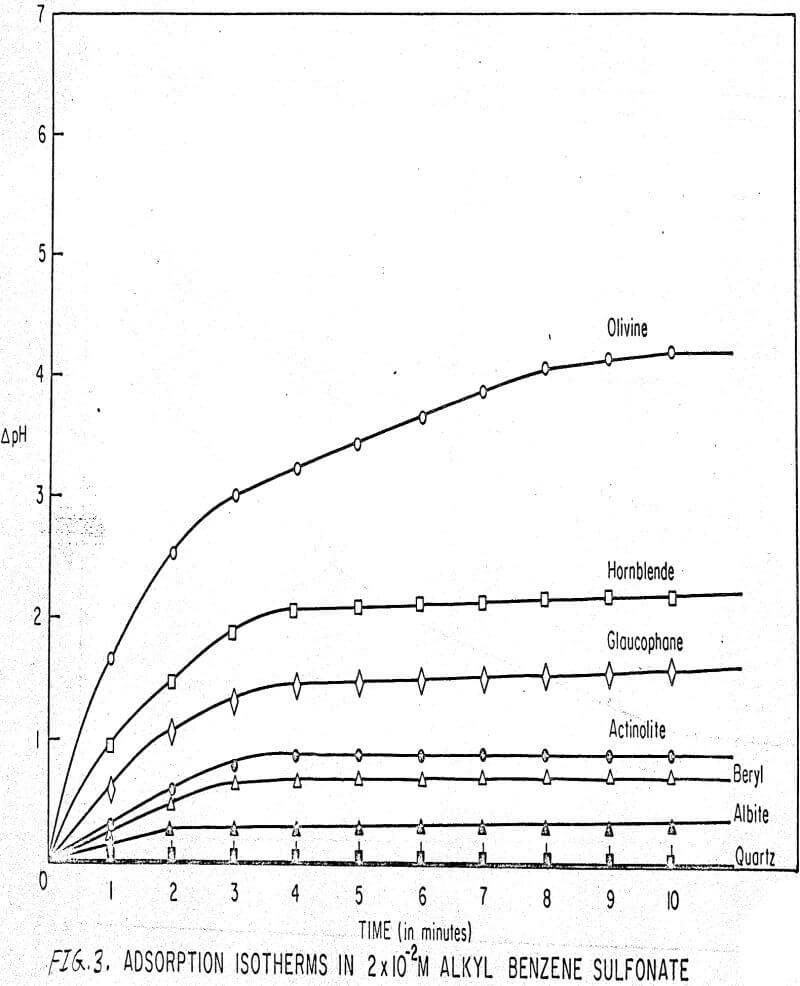
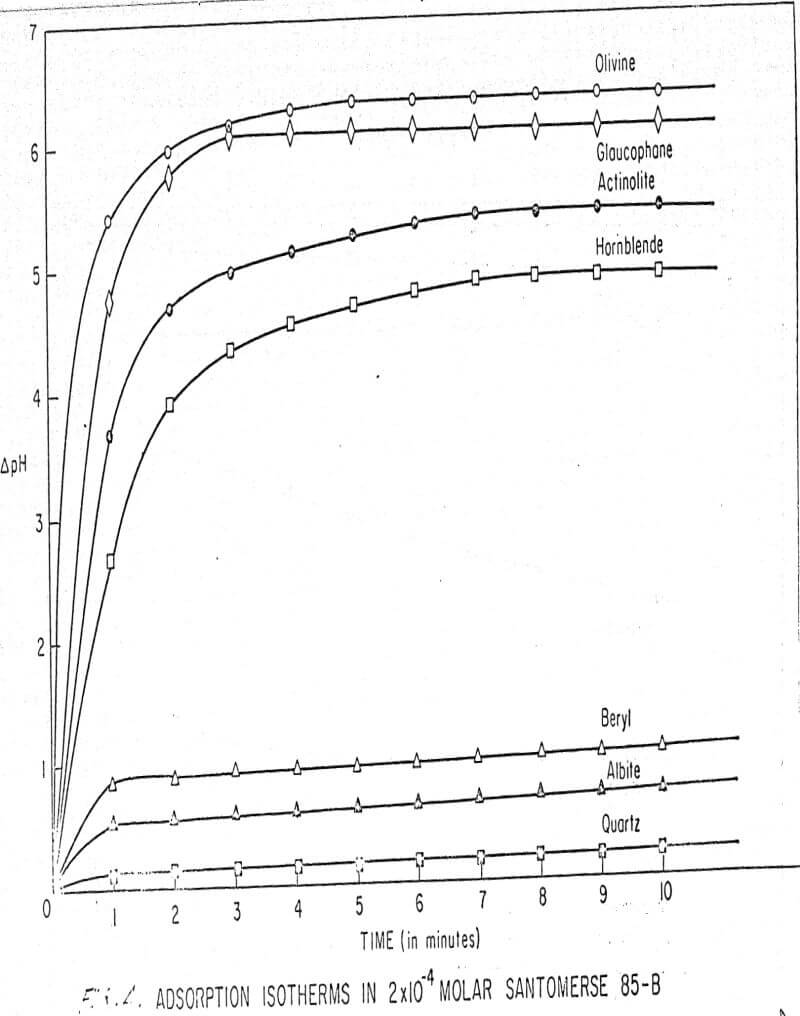
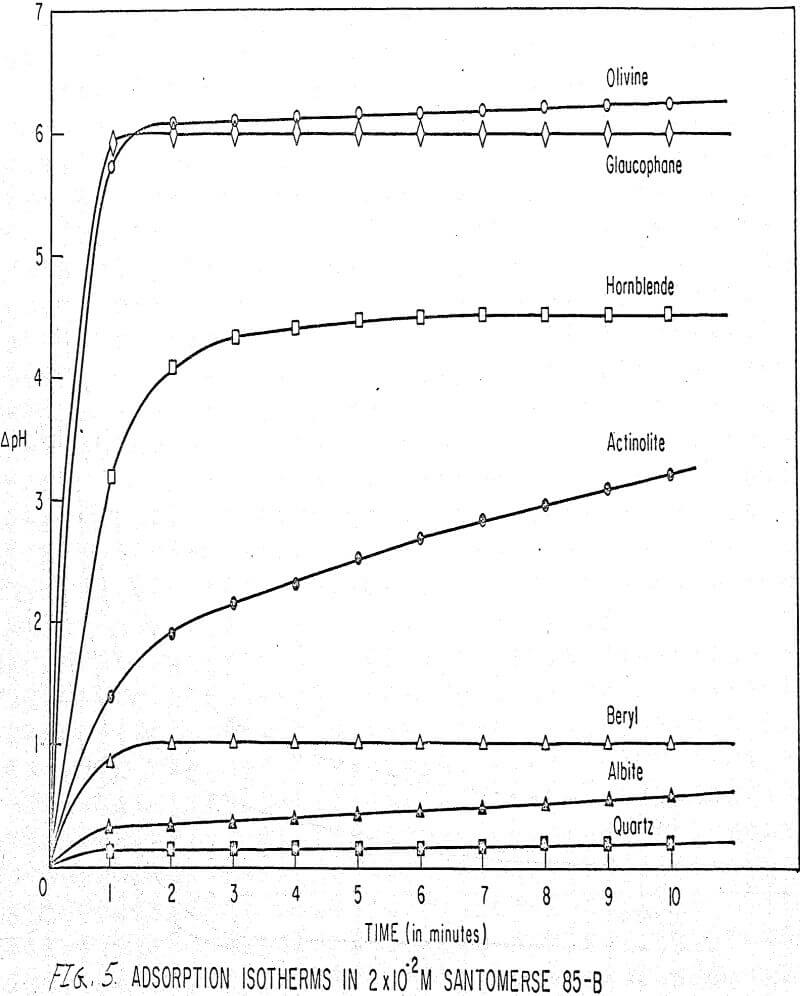
Results of these experiments are shown in Table 1 and Figures 1, 2, 3, 4, and 5. The curves follow equation (3), previously derived.
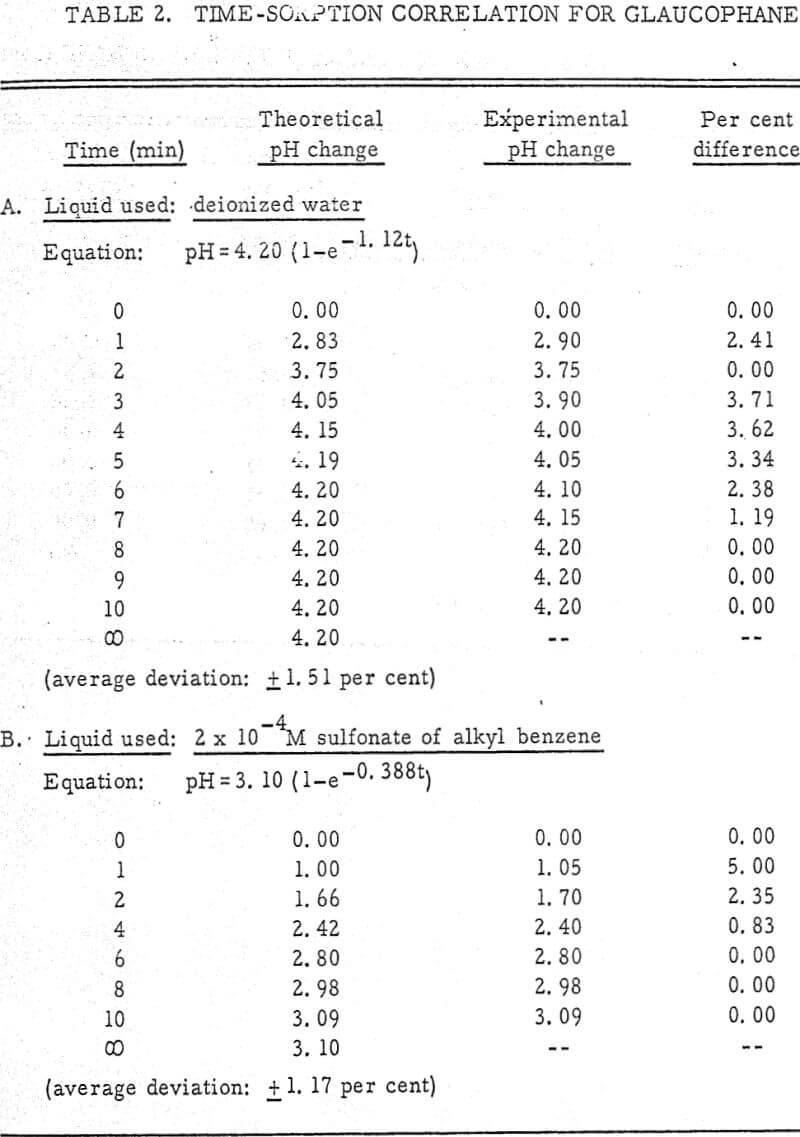
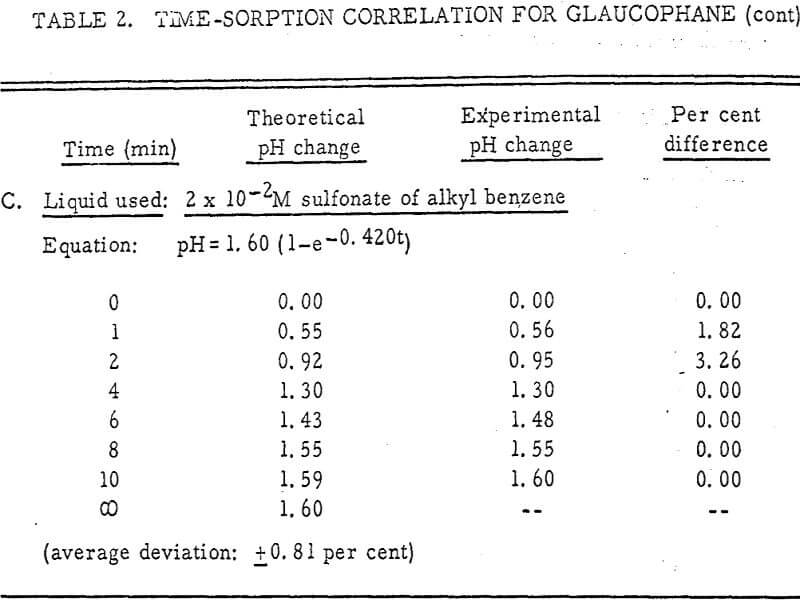
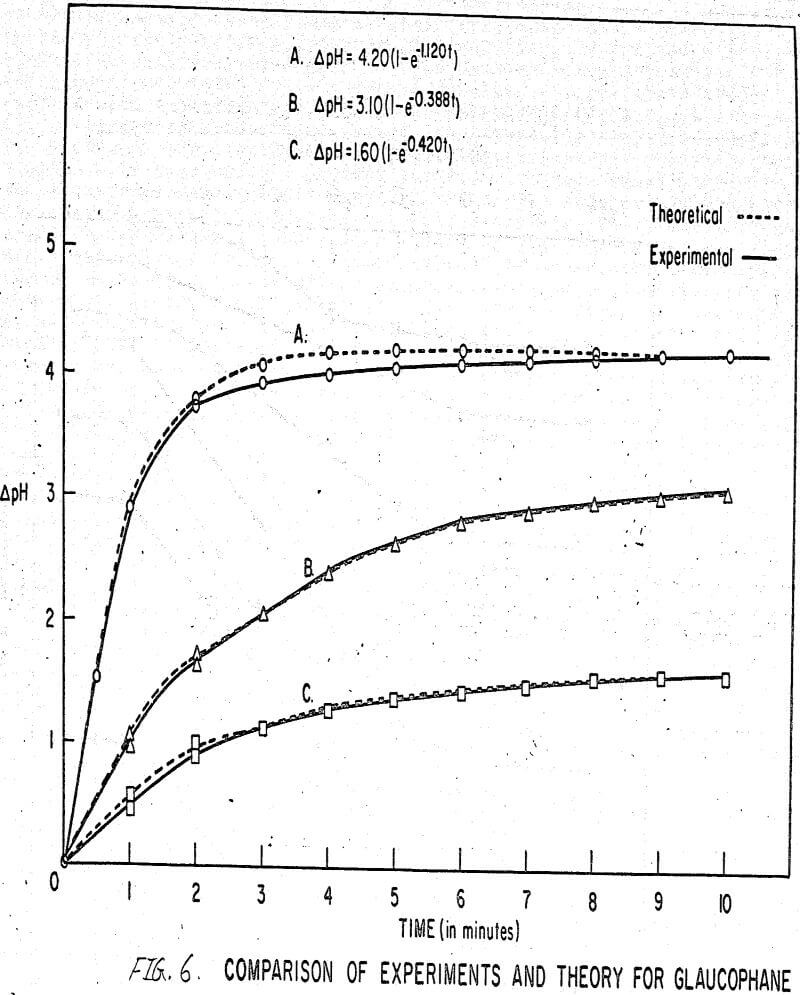
The results shown in Figures 1 to 5 were correlated with theoretical results obtained using equation (3). This correlation is shown for glaucophane for several concentrations in Figure 6. Table 2 shows the correlation for different times during the reaction. Error analysis of the correlation gives a mean deviation of ±1.2 per cent, which is well within accuracy limits. It is evident from Figures 1 to 5 that as the concentration of sulfonate is increased, the total change of pH decreases. This seems to indicate that the sulfonate is attaching to some of the metal cations present at the surface of the mineral, thus preventing the exchange of hydrogen for these cations.
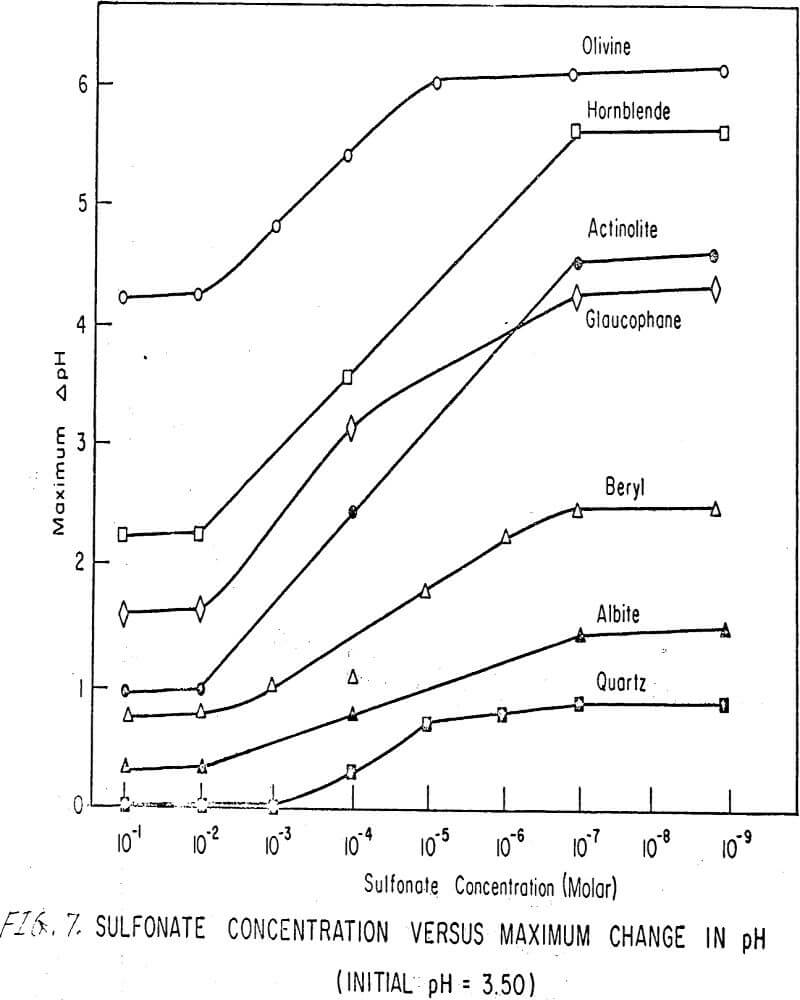
To further examine the effects of concentration on the total pH change, the net change of pH of each silicate mineral was determined as a function of sulfonate concentration using the sodium sulfonate of alkyl benzene. Results are shown in Figure 7, the plot of which can be divided into three main regions as follows:
Region A. This is the region where the concentration of sulfonate is so high that a minimum number of hydrogen ions are sorbed on the surface. This region extends, inmost instances, from concentrations of 10-²M up. The pertinent equation is
maximum ΔpH = constant…………………………………………………….(5)
Region B. In this region, the concentration of sulfonate is less than in the previous one; therefore, some hydrogen ion sorption can take place. As the sulfonate ion concentration decreases, the hydrogen ion sorption increases. A maximum sorption of hydrogen ions finally occurs at the lower end of this region.
Region C. This part of the plot is also a straight line; that is, equation (5) is satisfied. At such low concentrations (below 5 x 10 -7M) of sulfonate, the role of the sulfonate ion is minimal, and thus maximum hydrogen sorption occurs.
The sorption experiments demonstrate that the sulfonate ions can play an important role in reducing the amount of hydrogen ions that are exchanged for metal cations present on the mineral’s surface.
Discussion
The curves in Figures 1 to 5 satisfy the hypothesis postulated by Deju and Bhappu concerning the relation between hydrogen sorption and the oxygen-silicon ratio of the mineral.
It is known that as the oxygen-silicon ratio increases from quartz to the olivines, a greater percentage of the oxygen bonding power is available for bonding to cations other than silicon. Hence, with an increasing oxygen-silicon ratio, there is increasing oxygen-to-metal bonding. Upon the fracturing of a silicate mineral crystal, the oxygen-metal bond, which is almost entirely ionic in character, should break more easily than the stronger oxygen-silicon bond, resulting in a greater number of unsatisfied charges on the surface. Then, if the mineral is immersed in a liquid containing hydrogen ions, these negative charges should tend to be neutralized by hydrogen ions from solution, resulting in a change of the solution pH. Thus, an increase in the degree of sorption of hydrogen ions must occur as the oxygen-silicon ratio in the crystal structure increases. This phenomenon has been observed not only for water but also for the two sulfonates used at all concentrations of sulfonate ion.
Finally, the data of Table 1 show that the reduction in hydrogen ion sorption using Santomerse 85-b was less than for the sodium sulfonate of alkyl benzene. This behavior may be due to some or all of the following properties of the collectors:
- The average carbon chain of the sodium sulfonate of alkyl benzene has 15 carbons, while that of Santomerse 85-b has only 11.
- The activity of the sodium sulfonate of alkyl benzene is 99 per cent, while that of Santomerse 85-b is only 86 per cent.
- The molecular weight of the sodium sulfonate of alkyl benzene is 390, while Santomerse 85-b has a molecular weight of 339.
The influence of the carbon chain length and its configuration, impurities contained in the collector, and other physical and chemical properties of the collector on sorption characteristics are currently being investigated.
Sorption and Sorption Rate in an Amine System
A series of experiments was performed with one silicate mineral, olivine to examine experimentally the effect of aminium ions on sorption; that is to examine the relation between ionic strength and the constants Co and Cl. The apparatus and experimental procedure used were the same as for the sulfonate system.
Materials
Amine
The amine used was ARMAC TD (Tallow Amine Acetate), manufactured by Armour Chemical Company. This amine has a mean molecular weight of 328 and an average carbon chain of 17.4. Its activity is nearly 100 per cent. Concentrations of 10 -6M, 5 x 10 -5M, and 10-³M were used in the experiments.
Mineral
The mineral used in this experiment was an olivine having a forsterite composition. This sample was identical to the one used in previous experiments with sulfonates.
Procedure
The experimental procedure used was the same as that for the sulfonates.
Results
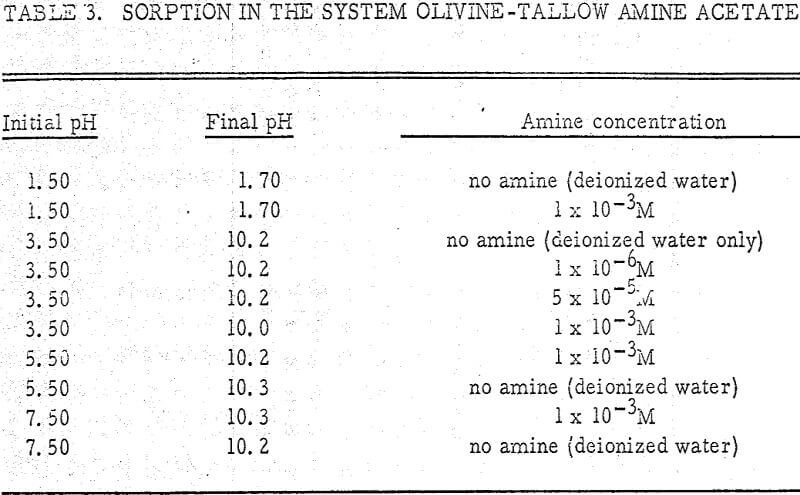
The initial pH was varied from 1.50 to 7.50. Since the amine breaks down at high pH values, no experiments were carried out above pH 7.50. Results are shown in Table 3.
From the table, it appears that the concentration of amine does not have an appreciable effect on the final pH. In fact, the sorption isotherm for olivine with an amine present is identical to that in deionized water (Figure 1). Thus, the concentration of aminium ion has no effect on the rate constants Co and Cl in equation (3).
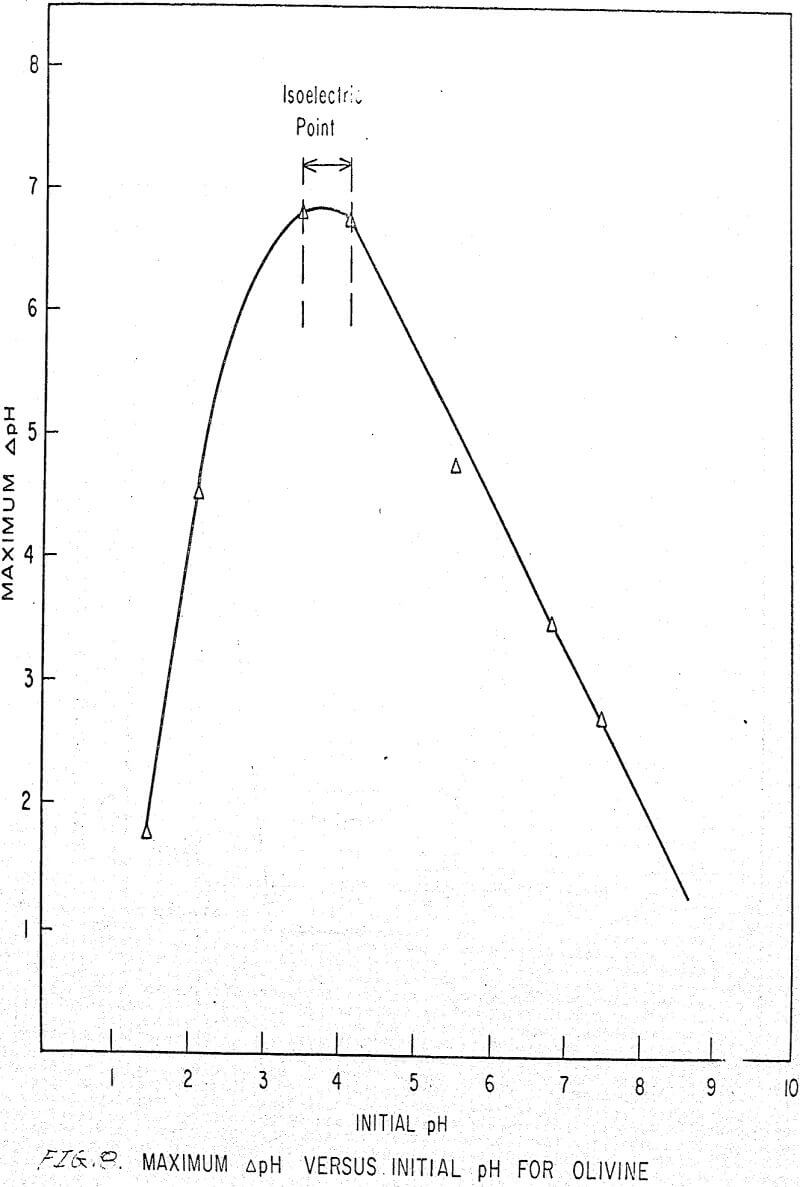
Also, if the change of pH in the presence of amine and in deionized water for different initial pH values is plotted, we obtain the curve shown in Figure 8, which is identical for deionized water and any concentration of amine.
This curve shows a sorption peak at pH 3.8. This is very close to the isoelectric point of forsterite (4.10) and confirms the hypothesis postulated by Deju and Bhappu that maximum hydrogen sorption occurs when the particle surface is neutral.
Thus, the experiments in this section seem to indicate that the aminium ion does not influence the sorption phenomenon under consideration.
Discussion
The results obtained in the previous section can be explained in terms of the isoelectric point (zero point of charge). The isoelectric point of a mineral is the condition existing when the number of positive charges on the surface exactly equals the number of negative charges on the surface. In our work, the potential determining ions are H+ and OH-, and we use pH as a measure of their concentrations. Thus, isoelectric point here refers to the point on the pH scale at which the surface is neutral.
The isoelectric point of olivine has been measured (Purcell and Bhappu, 1966) and found to be 4.10 (± 1.0 per cent). At pH values below 4.1, the surface of olivine is positively charged and above 4.1, the surface has a net negative charge.
Since below pH 4.1 the surface is positively charged, the amine, which is cationic in nature, cannot attach to the metal cations present at the surface of the mineral. Thus, below pH 4.1, the exchange of hydrogen ions for metal cations at the mineral surface is not affected by the presence of amine at any concentration.
Above the isoelectric point, the surface of olivine is negatively charged and the amine can attach to the surface of the mineral. Also above the isoelectric point of olivine, the concentration of hydrogen ions in the liquid is small. Consequently, hydrogen ion-metal cation exchange is small and an excess of metal cations exists at the mineral surface. Under these conditions, two things can happen:
- The aminium ion can exchange with metal cations at the surface or
- metal-amine complexes can be formed.
However, neither of these two, processes affects the exchange of H+ ions for surface cations, as shown by the experiments.
Ion-Exchange Experiments
To study the reaction occurring at the solid-liquid interface in more detail, a series of experiments was performed with several silicate minerals using sulfonate and amine solutions, as well as deionized water.
Samples of solution and solid were analyzed before and after each sorption experiment, using a Perkin-Elmer Model 303 Flame Spectrophotometer. Ions analyzed for were Na+, K+, Ca++, Mg++, and Fe. Solids were analyzed using an ARL Spectrographic Analyzer. Analyses for sulfonate and amine ions were carried out using a spectrophotometric procedure furnished by Shell Chemical Company.
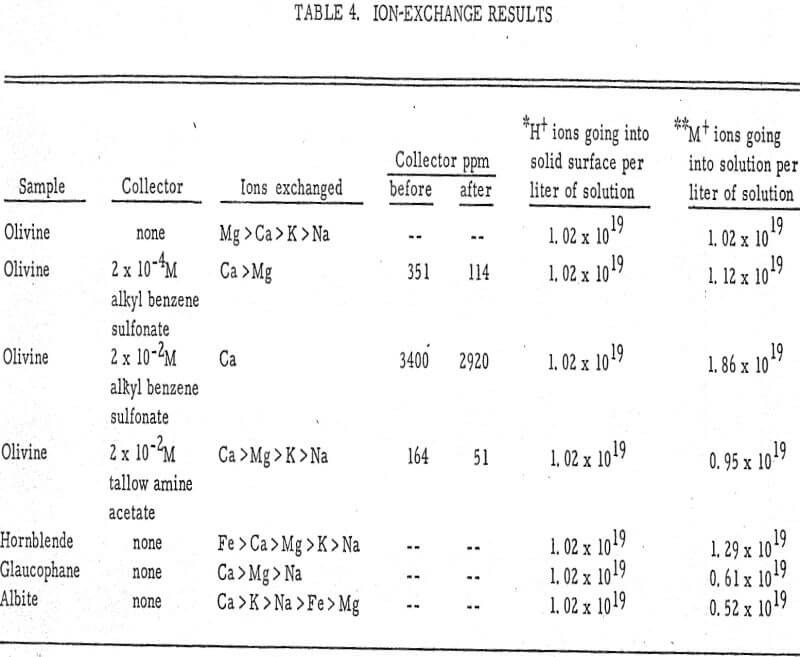
From the chemical analyses, the kinds of ions exchanged are known. These are shown in Table 4. Also shown is the number of H+ ions going onto the solid surface and the number of metal cations (M+) going into solution.
Discussion
Calcium and magnesium ions seem to be the ones most readily exchangeable, while sodium and potassium exchange comparatively less. This has been shown for organic resins (Dow Chemical Company, 1964) in which divalent ions are more tightly held by the resin than monovalent ions and trivalent ions more tightly than divalent ions. In the present experiment also, the order of preferential exchange is Ca++ > Mg++ > K+ > Na+. These metal cations exchange for H+ ions in the water solution following the general reaction
(pn)HCl + (pn)H2O + 2MpYn ↔ pM(Cl)n + pM(OH)n + 2nHpY
where M+ is the metal cation, Y- the anionic part of the silicate sample, p is the valence of Y, and n is the valence of M. This reaction has been discussed extensively by Deju and Bhappu. The number of H+ exchanged for M+ ions is very nearly equivalent, as shown in the last two columns of Table 4.
When sulfonate is present in solution, the reaction could be described on the basis of metal-collector complexes, probably of the type Mg(RSO3)x-.
In the presence of an amine, an amine-metal complex is probably formed since the reaction causes a considerable decrease in the amount of free aminium ion present in solution. However, experiments in this section seem to indicate that no exchange of surface cations for aminium ions occurs. In general, the experiments support the hypothesis postulated before that the collector seems to attach to some of the metal cations present at the surface of the mineral. The collector also seems to attach preferentially to divalent ions, such as Ca++ and Mg++, rather than to monovalent ions, such as K+ and Na+.
The extent to which the collector attaches to the mineral surface is a very important parameter and is the key to the froth flotation process.
Flotation of Silicate Minerals
On the basis of the previous discussion of sorption phenomena for silicate minerals, we are in a good position to study and explain the behavior of silicate minerals in froth flotation. Many investigators (Eigeles, 1960, 1961; Berlinskii, 1962; Choi and Oh, 1965) have studied the flotation properties of silicates. Purcell and Bhappu examined the correlation of silicate flotation with oxygen-silicon ratio. Patek and Biernat also attempted to correlate floatability with silicate structure.
To explain silicate flotation in terms of surface properties, a new index must be defined. This index, which we will call sorption index, is a function of the number of hydrogen ions going from the liquid onto the solid. It is given by the equation sorption index = K·ΔpH, where K is a constant.
Materials and Procedure
Two silicate minerals, olivine (maximum O:Si sharing) and quartz (minimum O:Si sharing) were used. They were dry-crushed to -48+65 mesh and run through the magnetic separator to remove any iron-bearing contaminants. All the samples were stored dry in clean polyethylene bottles.
The collectors used were sodium sulfonate of alkyl benzene (Monsanto) and tallow amine acetate (ARMAC TD). Solutions of these collectors of concentrations ranging from 10-²M to 10 -7M were prepared daily. Reagent grade HCl and NaOH were used for pH regulation. All flotation tests were carried out using one-half gram samples in a newly designed flotation cell (Purcell, 1965). Minerals were conditioned for five minutes and the aeration rate and volume were adjusted to give a flotation time of approximately five minutes. It was noted that in some of these tests an acidic pulp increases the pH as much as two or three units during conditioning. The pH after conditioning was used in presenting results of these tests.
Results
Olivine Group
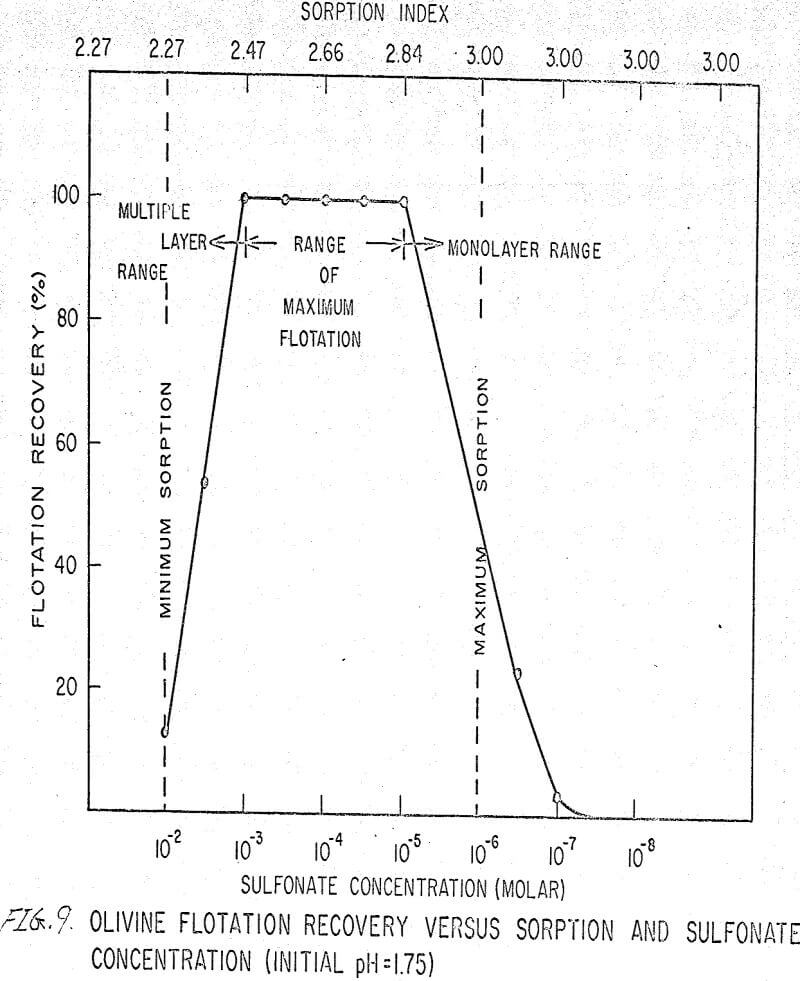
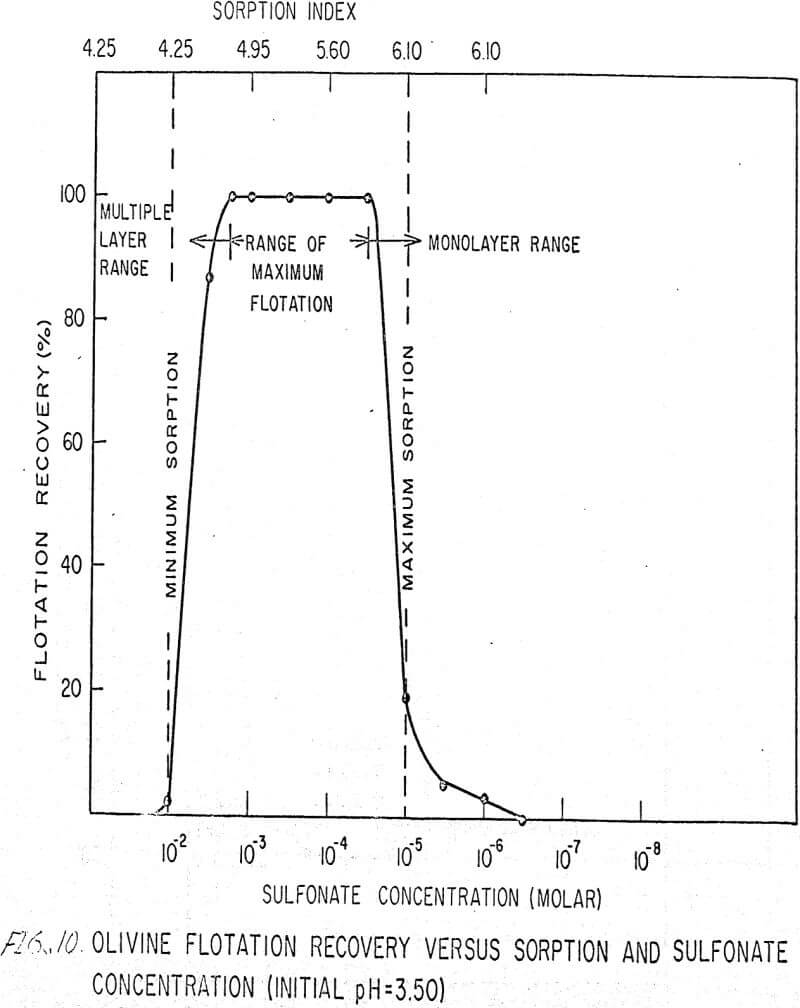
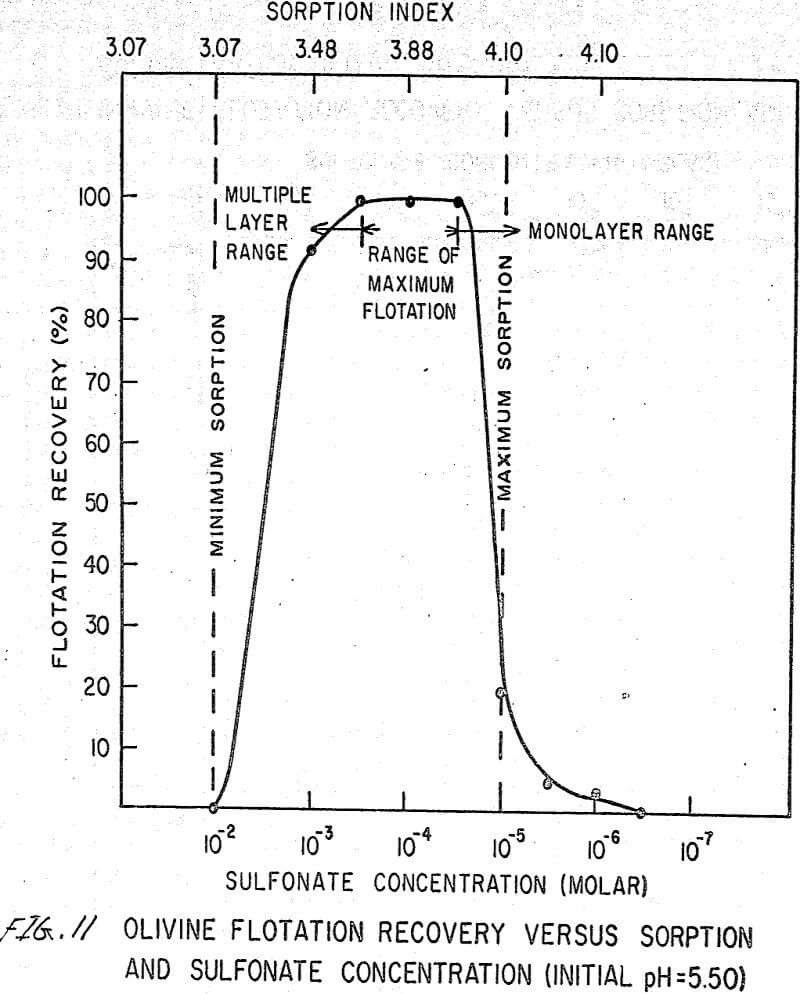
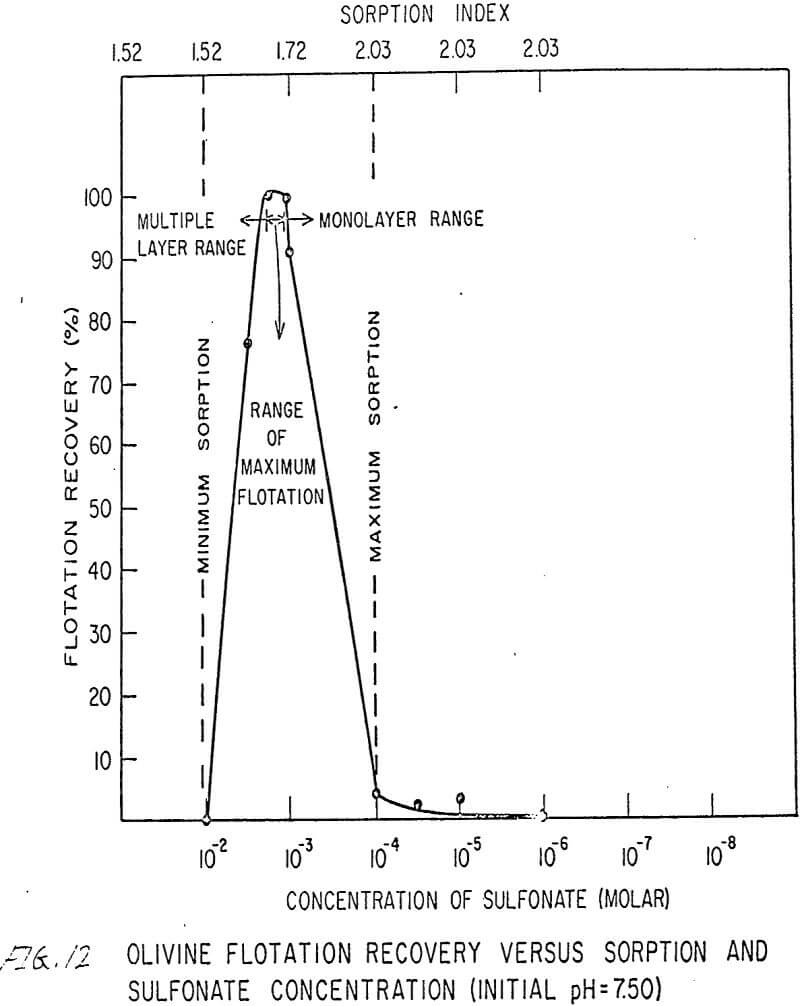
The flotation behavior of olivine using a sulfonate is shown in Figures 9 to 12. With a sulfonate collector, olivine floats strongly only in acidic pulps. The extent of the maximum recovery region increases as. the pH decreases up to the point where the solid begins to decompose. The isoelectric point of olivine is about 4.1. Thus, above this pH, the range of the maximum recovery region decreases.
Olivine is very soluble in concentrated HCl. Purcell and Bhappu showed that as the pH of an olivine pulp is lowered, the concentration of magnesium and iron cations increases, so that at a pH about 1.75, their concentration should be orders of magnitude higher than the concentration of collector. Also, the ion-exchange experiments showed that although magnesium is the predominant species in an olivine ({Mg, Fe)2SiO4), calcium ion exchanges to a much higher degree than magnesium in the presence of sulfonate.
This seems to indicate that some of the magnesium ions in the surface are forming an intermediate ionic form, such as Mg(RSO3)x-, which is then attracted to the surface.
If the flotation curves shown in Figures 9 to 12 are compared with Figure 7, it is clear that the area of maximum hydrogen sorption corresponds to the region of monolayer formation (Region I). The area of increasing hydrogen sorption corresponds to the region of maximum flotation (Region II). The area of minimum hydrogen sorption corresponds to the region of formation of additional layers where collector is in excess (Region III). An explanation of the phenomena occurring in these regions follows:
Region I. In this region, the concentration of sulfonate is not high enough to permit much flotation, and thus sorption index is a maximum because there are no interfering ions.
Region II. At the low-concentration end of this region, there are enough collector ions to give maximum flotation. Also, the number of collector ions is now enough to cause a decrease in the amount of hydrogen sorption. As the concentration of collector increases within this region, the surface coverage by the collector increases, thus lowering sorption index. At the high-concentration end of this region, maximum flotation is achieved but now, since the surface is pretty well covered, the degree of hydrogen sorption is a minimum.
Region III. In this region, sorption index is a minimum because there is an excess of collector. Also, a multiple layer of collector ions tied tail to tail is probably being formed within this region. Islands of collector ions also are likely to occur. Flotation therefore decreases as the concentration increases. Clearly, there is a correlation between the sorptive properties of olivine, the formation of multiple layers, and floatability with a sulfonate collector.
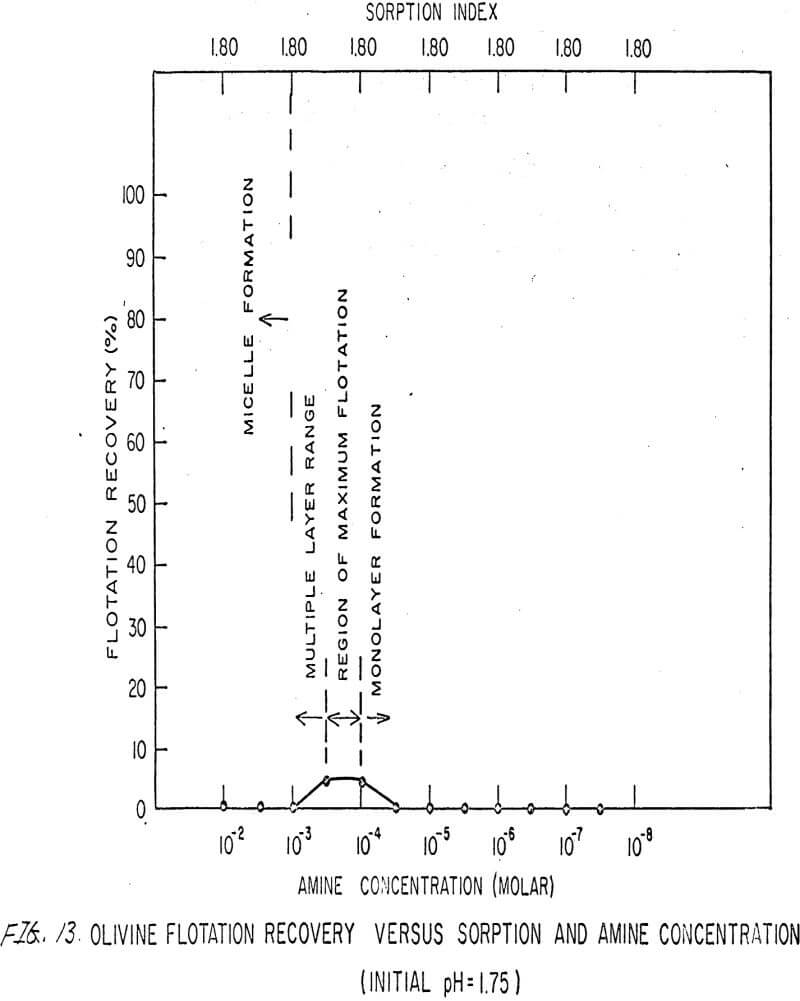
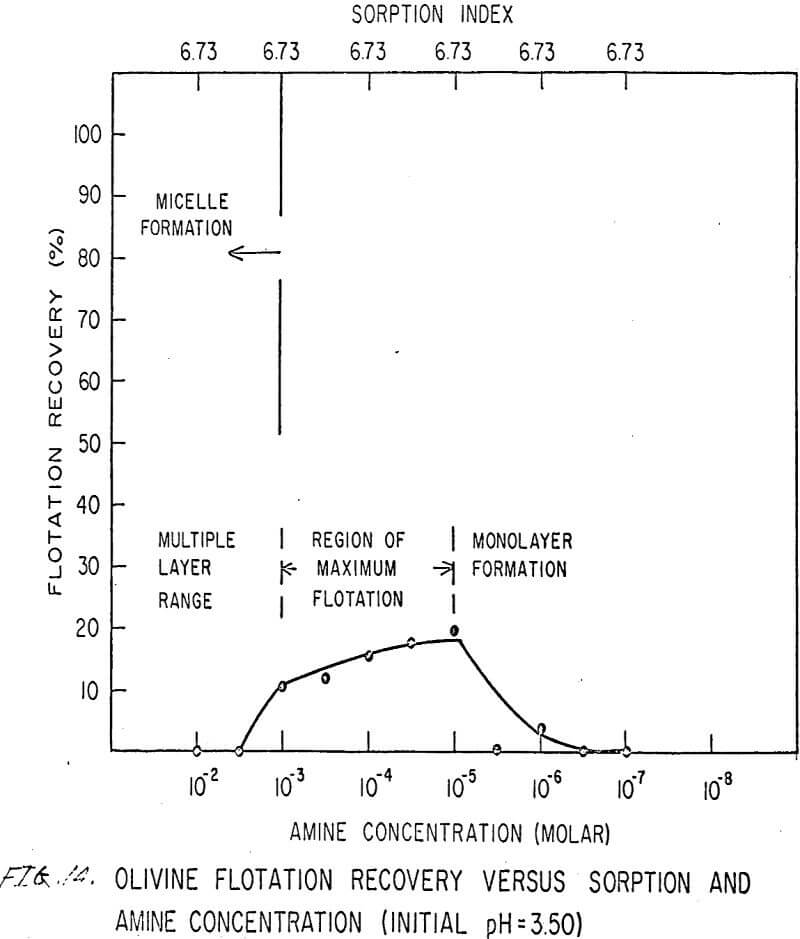
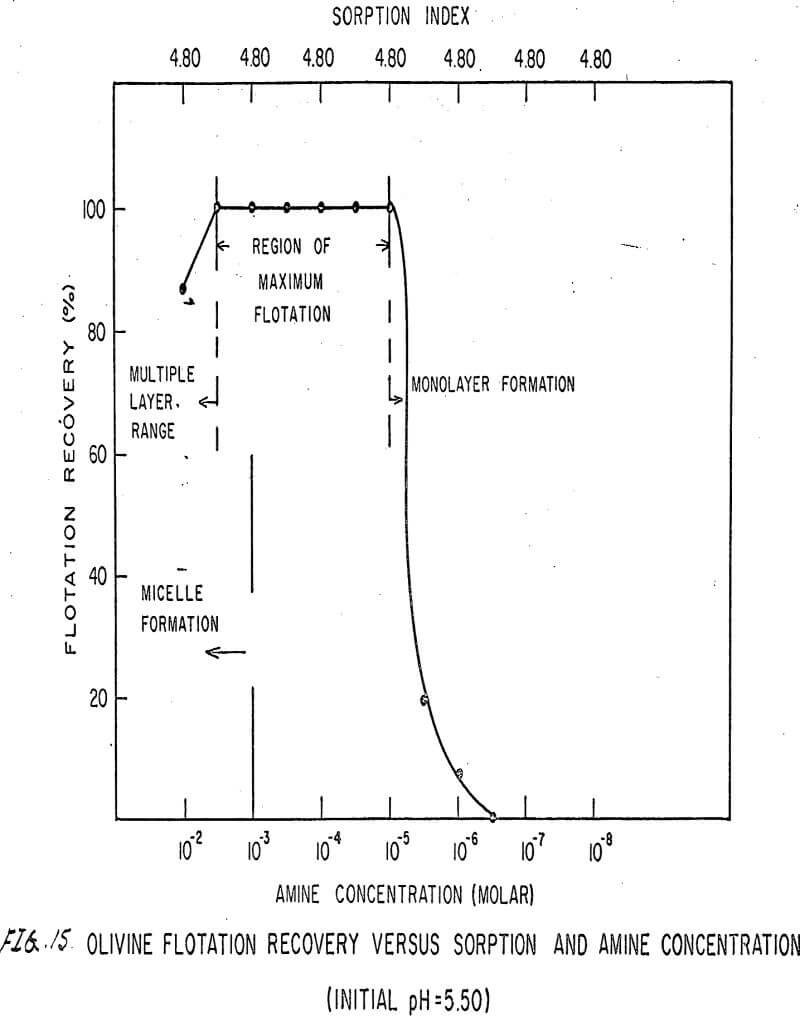
For ARMAC TD, olivine does not float well below the isoelectric point (Figures 13 and 14) because the surface has the same charge as the collector ion, and electrostatic repulsion occurs. As the pH increases above the isoelectric point, the collector can readily attach to the mineral surface, and flotation increases (Figure 15). Above pH 10.0, however, the amine breaks down and erratic results are obtained.
Also, amine does not affect sorption at the solid-liquid interface. The sorption index is constant for all concentrations of amine.
Nonetheless, there are again three regions in the flotation pattern corresponding to the monolayer region, the region of maximum flotation, and the region of multiple layer formation. These regions are less clearly defined than for sulfonates.
Quartz Group
The quartz used in the experiments had an isoelectric point of 1.7. Due to electrostatic repulsion, no flotation was possible above this pH using sulfonate collector.
The lack of flotation below the isoelectric point may be attributed to the absence of cations capable of reacting with the sulfonate.
With an amine (ARMAC TD), quartz floats well. However, the phenomenon is not explainable on the basis of sorption changes, since hydrogen sorption is a minimum for quartz. Quartz floats above the isoelectric point with an amine because of the negatively charged surface.
Summary
The change of pH after a time t has elapsed can be determined by the equation,
![]()
where Co and Cl are experimentally determined constants. This equation will hold for any liquid phase. However, in comparing sorption isotherms obtained with this equation for different liquids, the effect of ionic concentration in the liquids must be considered.
Experimental results of sorption experiments with glaucophane were correlated with the above equation. Error analysis of the correlation gives a mean deviation of ± 1.2 per cent, which is well within accuracy limits.
As the sulfonate concentration is increased, the exchange of hydrogen ions in the liquid for metal cations in the surface of the solid decreases. This seems to indicate that the sulfonate is attaching to some of the metal cations present at the surface of the mineral, thus preventing the exchange of hydrogen for these cations.
The net change of pH of each silicate mineral was plotted as a function of sulfonate concentration using a sodium sulfonate of alkyl benzene. This plot has three regions: A, minimum H+ sorption; B, varying H+ sorption; C, maximum H+ sorption.
The degree of reaction depends directly on the oxygen-silicon ratio of the silicate structure, being greatest for the olivines. This dependence is not affected by the presence of collector ions in the liquid.
The concentration of amine does not have an appreciable effect on H+ exchange for M+ ions. In fact, the isotherm for olivine with an amine present is identical to that in deionized water.
Maximum sorption of H+ ions occurs at the isoelectric point of the mineral. The presence of collector does not affect this phenomenon.
Aminium ion can attach to the surface of a silicate above its isoelectric point but not below, since below this point electric repulsion occurs.
Ion-exchange experiments with silicate minerals indicate the following order of preferential exchange:
Ca++ > Mg++ > K+ > Na+
These metal cations exchanging for H+ in solution follow the general reaction
(pn)HCl+ (pn)H2O + 2MpYn ↔ p M(Cl)n + p M(OH)n + 2nHpY,
where M+ is the metal cation, Y- is the anionic part of the silicate sample, p is the valence of Y, and n is the valence of M.
A sorption index to correlate the sorptive properties of olivine, the formation of multiple layers, and the floatability with a sulfonate collector was defined.
The flotation behavior of both olivine and quartz in the presence of an amine was explained in terms of sorption and electrokinetic properties of the minerals.
Experiments showed that, in general, to explain floatability of silicate minerals, the following factors must be considered:
- the value of the sorption index and its functional relation to sulfonate concentration;
- the electrokinetic properties of the mineral;
- the stability and formation of metal-collector complexes;
- the number and kind of ions exchanged at the solid-liquid interface; and
- the preferential attachment properties of the collector in use.