Table of Contents
Laboratory experiments were conducted with cornstarch to study the effects of duet concentration, moisture, particle size, treatment with oil, and admixture of calcium carbonate on the dust-explosion hazard.
One series of tests were made on five size fractions of starch and on samples with four different moisture contents, all prepared from a single batch. Within the range of the experiments covered, the test data indicate the following:
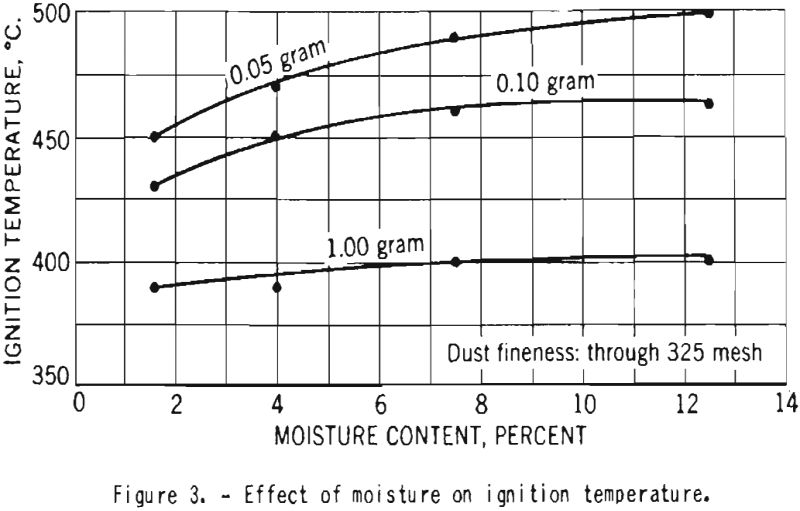
- The ignition temperature decreased with increase in the concentration of the duct cloud. The ignition temperature of starch containing 12.5 percent moisture was only slightly higher than for starch with 1.6 percent moisture. There was a definite increase in ignition temperature with increase in particle size. This is in agreement with earlier data obtained in experiments on other dusts; it does not support the data reported for starch by Costa and Costantinides. The minimum ignition temperature of dust clouds of dry, finely divided cornstarch from this batch is about 390° C.
- The minimum energy required for ignition of duct clouds increased with the moisture content and also with increase in particle size. The minimum explosive concentration was affected similarly.
- Increase in moisture content and in particle size “both caused a reduction in the maximum pressures and the rates of pressure rise produced by starch explosions.
Mixing a small percentage of edible oil with the starch reduced its disporsibility and thereby its explosibility. However, when dispersed in air oven the oil-treated starch was capable of producing strong explosions.
Addition of calcium carbonate to starch reduced the case of ignition and the violence of the experimental explosions. To prevent ignition of duct clouds by electric sparks, about 60 percent carbonate was nodded in the mixture. To prevent ignition by hot surfaces, the carbonate content had to be increased to 90 percent; the required proportion is probably the same in the presence of open flames and other strong igniting sources.
In conclusion, the investigation indicates that a high moisture content in the starch reduces its explosion hazard but does not eliminate it completely, and that starch consisting of coarse size fractions is much less explosive than finely divided starch. However, it should be remembered that in practice nearly all starch contains a fair proportion of fine particle. The explosion hazard of starch can also be reduced in some measure by oil treatment and by the addition of calcium carbonate.
Thin investigation and past experience serve to emphasize that in handling large quantities of powdered starch, strict precautions are necessary to eliminate all igniting sources, to prevent the formation of dust clouds, and to provide explosion pressure relief vents in the plant.
Review of Work by Costa and Costantinides
A pertinent study on the ignition temperature of starch dust clouds has been published by Costa and Costantinides. As this report is not readily available to readers in the United States, it is briefly abstracted here.
The authors define the ignition or autoignition temperature as the minimum temperature at which a substance ignites spontaneously in air at atmospheric pressure. The experiments were conducted in a specially designed apparatus consisting essentially of a 600 cc. spherical glass ignition chamber placed inside a thermostatically controlled double-walled cabinet that contains electrical resistance-type heating elements. The dust is dispersed into the spherical glass vessel by dry compressed air, and it ignites if the temperature of the walls and air are sufficiently high.
Most of the work was done on cornstarch, but some studies were made also on rice starch, potato starch, a few wheat-flour and grain samples. The effects of the following variables on the ignition temperature were evaluated: (1) Dust concentration, (2) percentage of impurities, predominantly of protein character, (3) moisture content, (4) modification in chemical composition resulting from prolonged heating at temperatures between 100° and 200° C (5) particle size. Four size fractions wore studied, namely, 215 to 160 microns, 160 to 95 microns, 95 to 60 microns, and minus (or through) 60 microns.
The lowest ignition temperature of cornstarch was found to be 360° C. As the dust concentration was increased from 0.025 to 0.10 gram per liter, the ignition temperature decreased 10° to 20° C. for fine particles and up to 50° C. for coarser starch. Increase in the protein content of starch was accompanied by a small increase in the ignition temperature. There was little difference between starches with moisture contents of 0.67 and 5.66 percent, respectively, but further increase in moisture, up to 12.11 percent, resulted in about 12° C. rise in the ignition temperatures. Preheating cornstarch at temperatures up to 170° C . had little effect, but preheating to higher temperatures raised the ignition temperature.
The experiments with starch of four different size fractions gave surprising results. As the particle size was reduced, the ignition temperature of the dust clouds in the air increased. This is the reverse of what was expected and had been found in experiments with numerous dusts by other investigators. The values for the four size fractions mentioned above were 362°, 377°, 390°, and 413° C. at a dust concentration of 0.10 gram per liter. Costa and Costantinides recognized the anomalous character of this result and repeated the experiments with a few samples of bran dust and wheat flour. They found again that the ignition temperature increased as the size of the dust diminished. As a possible explanation for these findings the authors suggest that whereas, in case of metal powders, ignition and explosion are the result of direct and instantaneous oxidation of the metal, the case and rate of oxidation being a direct function of the total particle surface exposed to the air, in the case of organic material such as starch it is not the substances themselves that are rapidly oxidized, out rather the volatile combustible compounds that form as a result of pyrolytic processes that occur upon heating to higher temperatures. They suggest further that in their experiments when large dust particles are dispersed in the ignition chamber, the volatile combustible that is formed attains an explosive concentration more rapidly and at a lower temperature than is tho case in a dust cloud of finer particles.
Description of Tests
The apparatus used in the present test work vas essentially that described in Bureau of Mines Report of Investigations. Studies were made of the ignition temperature of cornstarch dust clouds in air, of the minimum energy required to ignite dust clouds by electric sparks formed by condenser discharge, of the lower explosive limit or minimum explosive concentration, and of pressures and rates of pressure rise produced by explosions in a closed test bomb.
Very recently dust dispersion in the 1.23-liter (75-cubic inch cylindrical test bomb was improved. As a result, the experimental explosions of the starch samples have developed much higher pressures at higher rates than had been measured and reported previously, The compressed air used for dispersing the dust was formerly stored in a 1.3-liter (80-cubic inch) tank at a pressure of approximately 70 cm. of mercury (13.5 pounds per square, inch, gage); in the recent experiments, tanks of 50 to 200 cc. (3 to 12 cubic inches) capacities were used, and the air pressure ranged up to 100 pounds per square inch.
The effects of the following variables were investigated: Concentration of dust cloud; moisture content of dust; particle size of dust; addition of a small percentage of oil; and admixture of calcium carbonate with the starch.
Test Samples
The particle-size studies were made on dried starch (1.6 to 1.8 percent moisture) of the following size ranges: 65 to 100 mesh (Tyler scale), 100 to 150 mesh, 150 to 200 mesh, through 200 mesh, and through 325 mesh. The corresponding average particle diameters are about 178, 126, 89, 37, and 22 microns.
As received, the starch contained approximately 12.5 percent moisture. To study the effect of moisture, a portion of the finest size fraction (100 percent through 325 mesh) was dried for varying periods at 75° c., and samples were prepared with moisture contents of 7.5, 4.0, and 1.6 percent. In addition, portions of the coarser fractions were dried until their moisture contents were reduced to 1.6 – 1.8 percent.
The oil-treated cornstarch used in one series of tests contained 0.58 percent edible oil. Its exact particle-size distribution could not be determined, because the dust particles stuck to the sieve. During drying, a small amount of starch was kept at 75° C. for 24 hours and lost 5.6 percent weight, presumably as a result of moisture loss. The explosibility tests were made on the undried starch.
The cornstarch used in tests with calcium carbonate contained 10.4 percent moisture, and 98 percent of the particles were finer than 200 mesh. The corresponding values for the carbonate were 0.3 percent moisture and 99 percent through 200 mesh. Tests were first made on mixtures of undried starch with the following percentages of added calcium carbonate: 0, 10, 20, 40, 60, 80, 90. Later, similar mixtures were prepared and tested with dried starch, which contained 1.3 percent moisture.
Results of Investigation
The test data are presented in a series of graphs and tables grouped according to variables whose effects on the explosibility of cornstarch were investigated.
As some of the measurements were made in metric units, it is desirable to indicate the English equivalents of the metric quantities used in this report.
1 gram weight = 0.0353 ounce, avoir.= 0.0022 pound, avoir.
1 liter = 1,000 cubic centimeters (cc.) = 0.001 cubic moter = 61.023 cubic inches = 0.035 cubic foot
1 mg. per liter = 1 gram per cubic meter = approximately 0.001 ounce per cubic foot
The minimum energy required for ignition of dust clouds by electrical sparks is expressed in joules. A joule is a unit of electrical energy equivalent to the work expanded per second by a current of 1 ampere flowing through a resistance of 1 ohm,
1 joule = 0.00095 British thermal unit (mean) = 0.00028 watt-hour
1 micron = 0.0001 cm. = 0.0000394 inch.
The widths of the square openings of the Tyler sieves that were used for separating the cornstarch into several size fractions are given below:
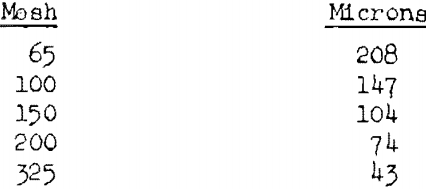
Effect of Dust Concentration
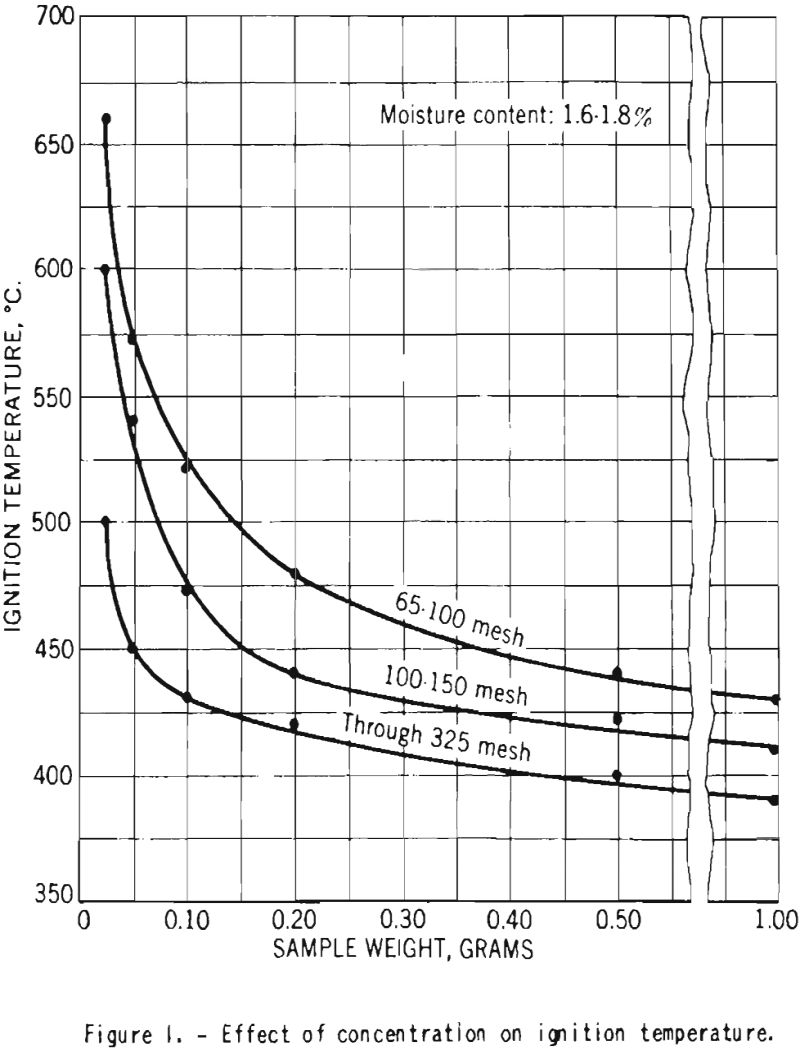
Figure 1 shows the effect of the weight of dust sample on the ignition temperatures of dust clouds of dry cornstarch of throe different sizes. As the sample weight was increased from 0.025 to about 0.20 gram, the temperature decreased rapidly, but further increase in weight had lesser effects.
These tests were conducted by dispersing the duet vertically downward through a cylindrical furnace open at the bottom; the exact concentration of the duet in the air is not known. The air used for dispersion is contained initially
under pressure (up to 50 cm. of mercury, gage) in a 500-cc. spherical glass vessel.
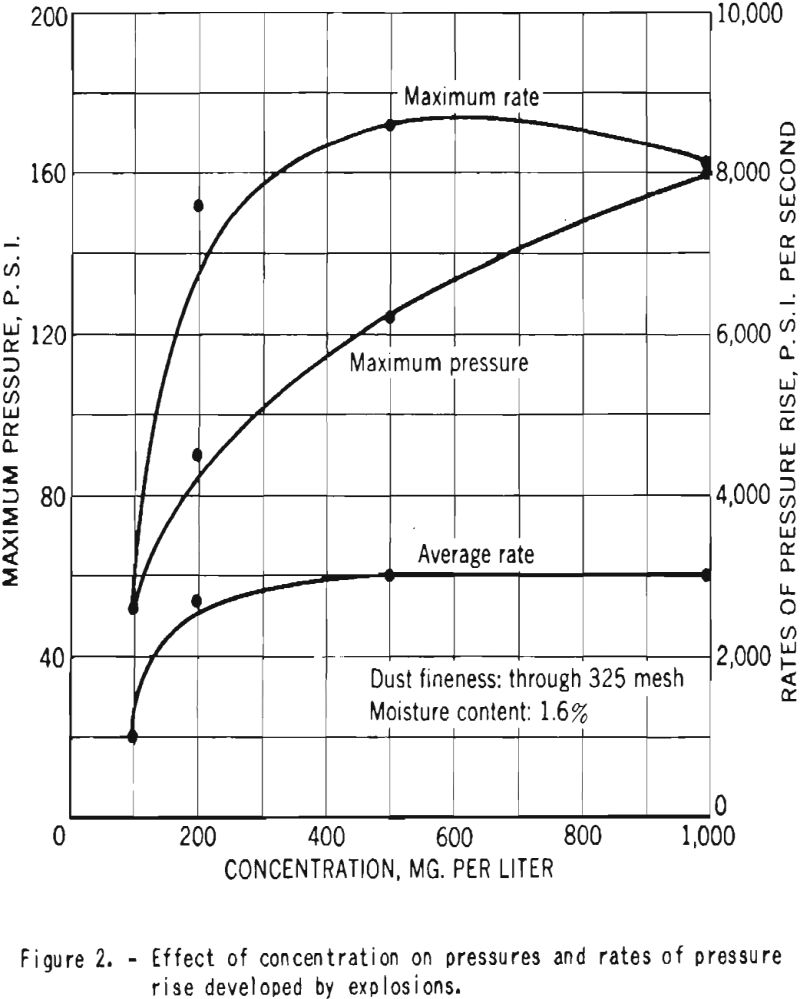
Figure 2 shows the variation with dust concentration of the maximum pressure and rates of pressure rise produced by explosions of dust clouds of finely divided dry cornstarch in air. As can be seen, the ratios reach maximum values within the range of concentrations tested, but the pressure shows a continuous increase, and its maximum value would be attained only at higher concentrations.
Effect of Moisture
Figure 3 illustrates the variation of ignition temperature with increase in moisture content of starch from 1.6 to 12.5 percent. For dust clouds of high concentration the effect was quite small, but in tests with samples of 0.05-gram weight the ignition temperature was increased by 50° C. as the moisture content increased.
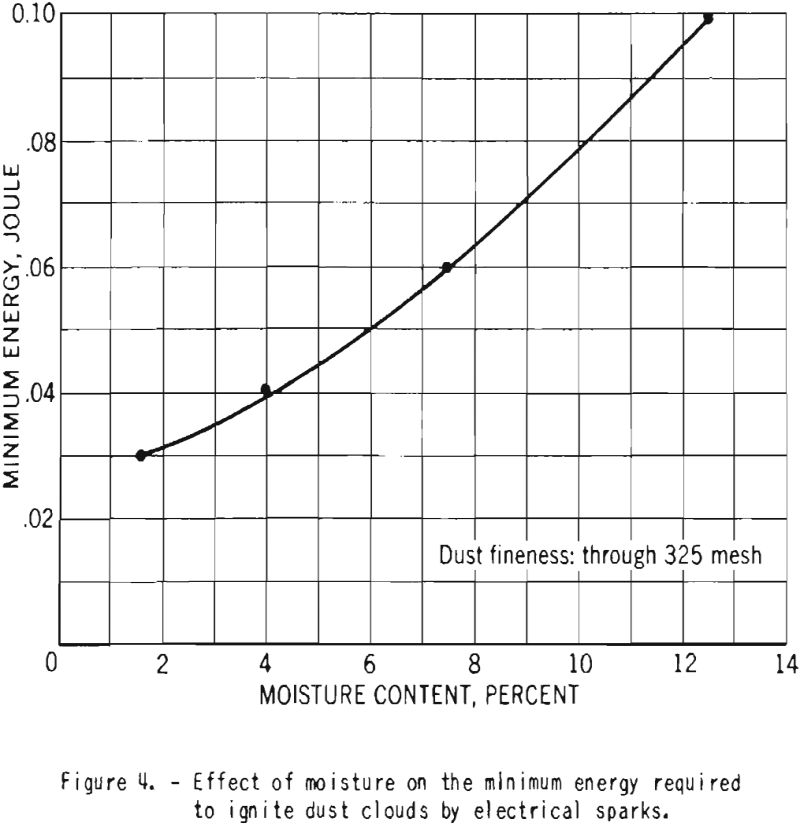
The minimum electrical energy required for ignition of duet clouds of fine cornstarch increased from 0.03 to 0.10 Joule as the moisture content was raised from 1.6 to 12.5 percent. This is illustrated in figure 4.
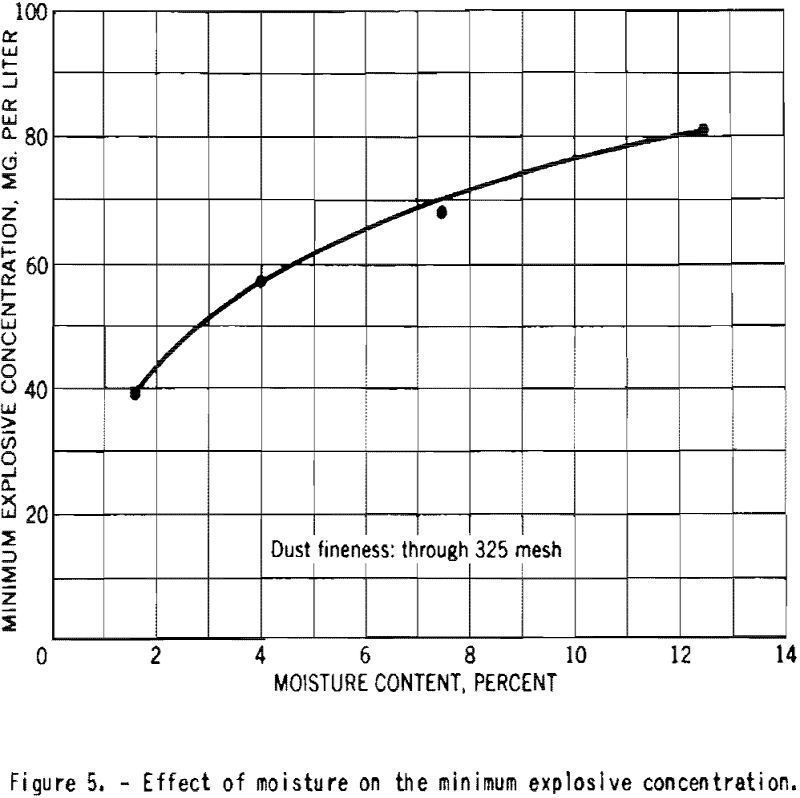
The minimum explosive concentration of finely-divided starch was approximately doubled as the moisture content increased, as shown in figure 5.
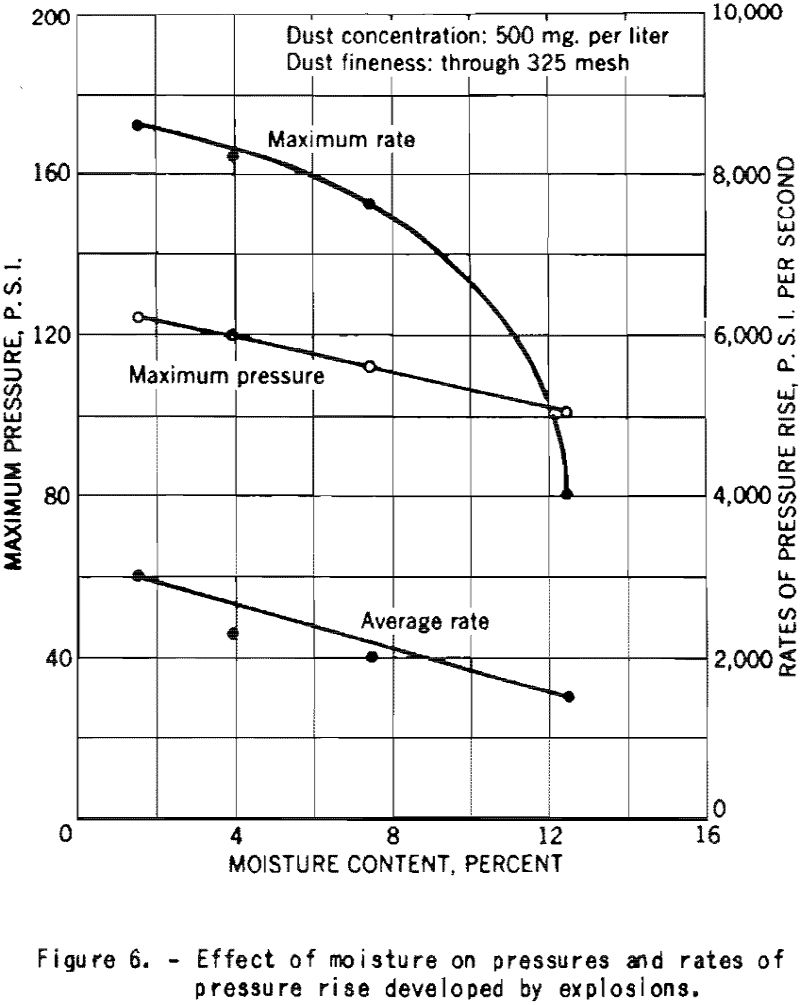
Figure 6 shows the decrease of the maximum explosion pressure and the ratios of pressure rise with increase in the moisture content of finely divided cornstarch. Those experiments were made at a dust concentration of 500 mg. per liter, or 0.500 ounce per cubic foot.
Effect of Particle Size
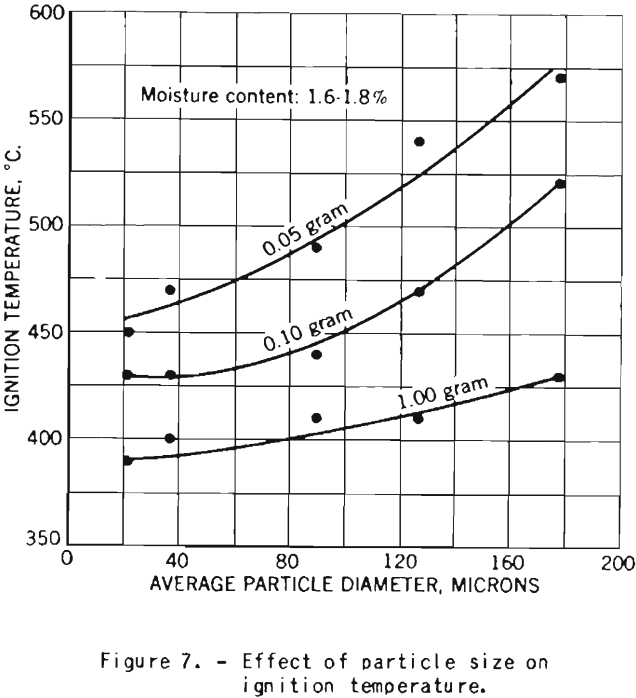
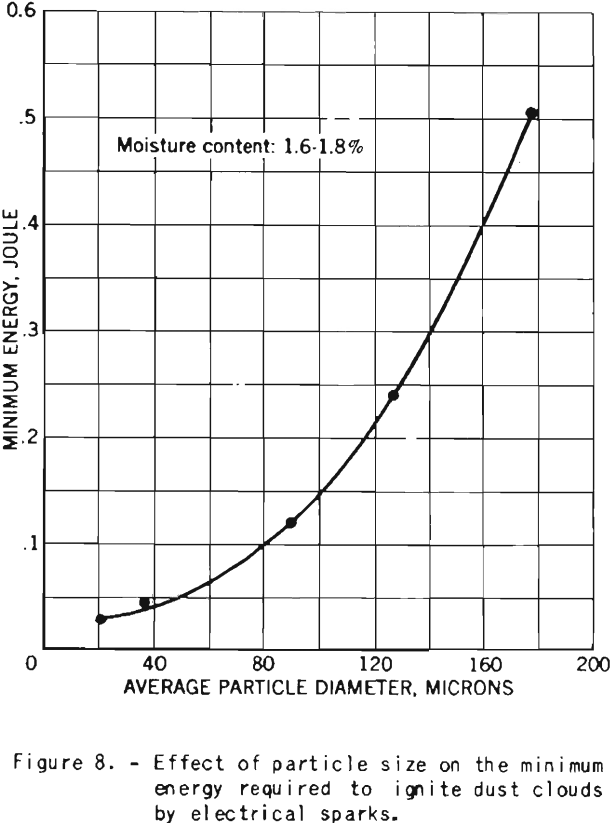
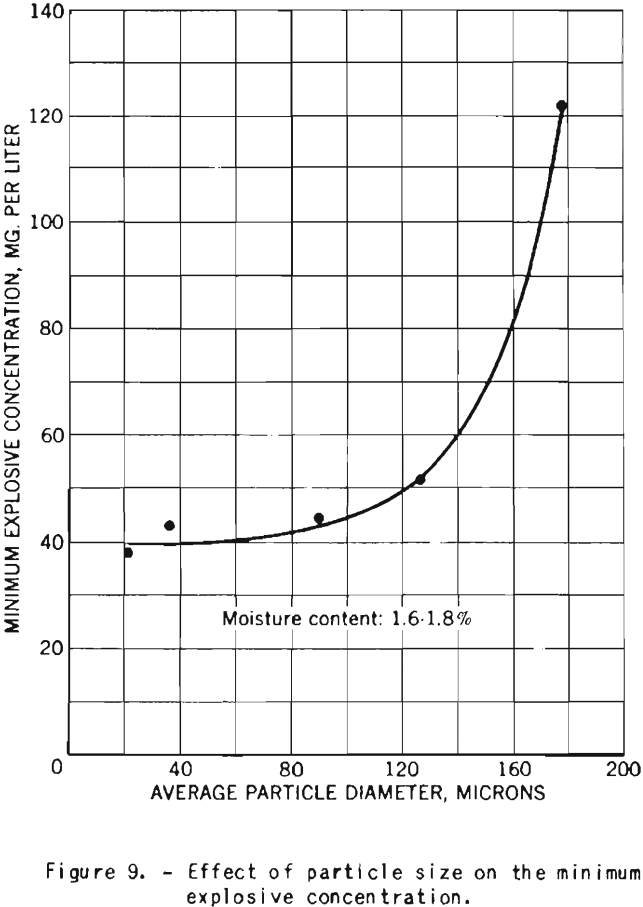
The tests on the effect of dust fineness on the explosive properties of cornstarch were made with starch having a moisture content of 1.6 to 1.8 percent.
Figure 7 shows the increase in ignition temperature with increase in particle diameter. The effect is more pronounced for dust clouds of small density than for samples of higher concentration.
The minimum electrical energy required for ignition of dust clouds increase rapidly as the particles become coarser, as shown in figure 8.
The minimum explosive concentration of dust clouds is approximately the same for particles of 20 to 90 microns diameter, but further increase in size results in rapid increase of the lower explosive limit, as illustrated in figure 9.
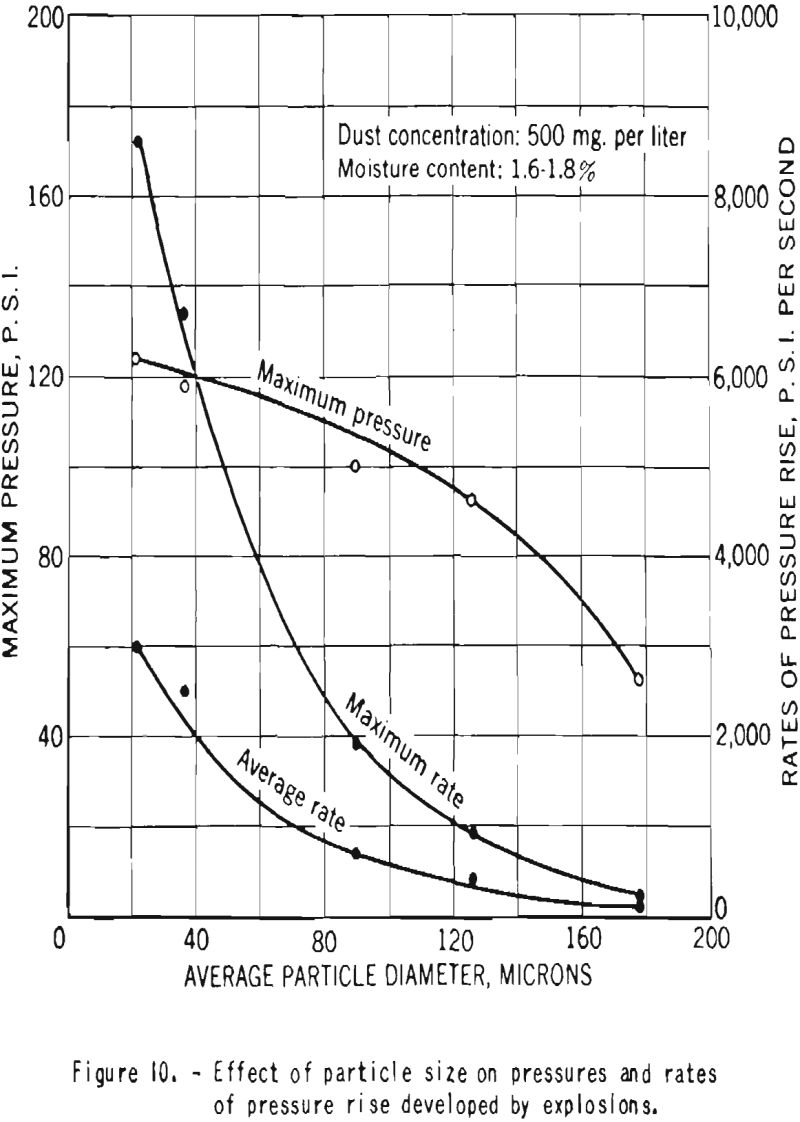
Increase in particle size was found to have a marked effect on the pressures and rates of pressure rise produced by dust explosions, as shown in figure 10. However, even dust cloud of cornstarch of 65- to 100-mesh fineness (average particle diameter about 178 microns) produced explosions sufficiently strong to cause great structural damage.
Explosibllity of Oil-Treated Cornstarch
The sample used in those tests was reported to contain 0.58 percent edible oil, One objective of this oil treatment is said to be the reduction of the case of dust formation and therefore indirectly of the hazards of explosions. It was noted that the dust clouds formed somewhat loss roadily than with untreated starch, but there was no difficulty in igniting the dust after it had been dispersed into the air. The test data are summarized in table 1.
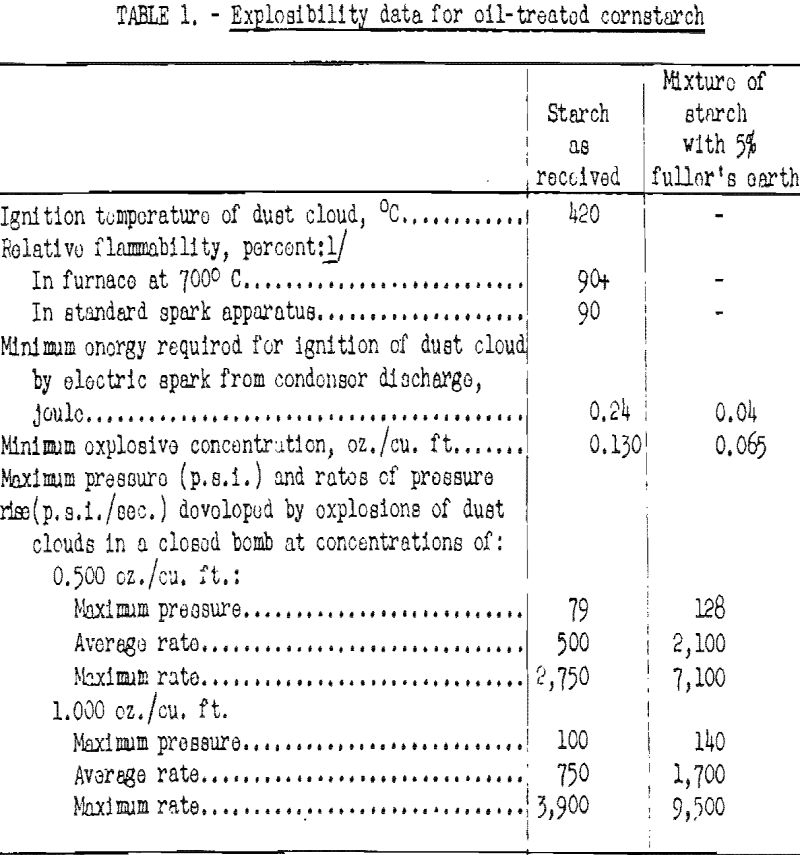
A few additional tests were conducted with a mixture of the oil-treated starch and 5 percent inert fuller’s earth of through 200-mesh fineness. The purpose of this addition was to reduce stickiness and promote dispersion of the starch. These test data are also given in table 1. Dust clouds of the mixture formed with greater case, required less energy for ignition, had a lower minimum explosive concentration, and produced higher explosion pressure and rates of pressure rise than clouds of starch without the fuller’s earth. These results indicate that the oil treatment had a beneficial effect in reducing the explosibility of starch, but even the oil-treated starch constitutes an important explosion hazard if dispersed in air.
Effect of Addition of Calcium Carbonate
One method of reducing the explosion hazard of starch that has been under investigation in the manufacture of candy is to incorporate inert powders with the starch used for dusting and molding. Among the compounds tested is food-grade calcium carbonate.
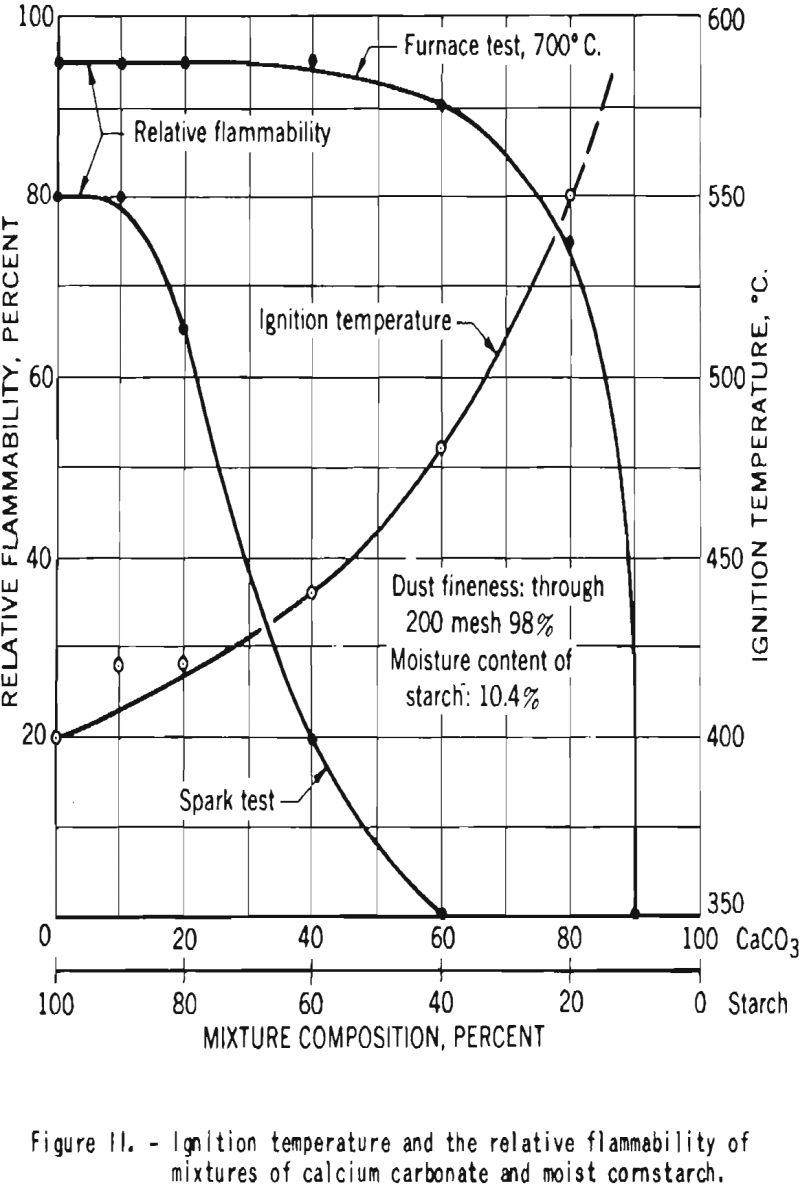
In one series of tests made at the Bureau of Mines, mixtures of starch with 10.4 percent moisture and calcium carbonate were prepared. The samples contained, respectively, 0, 10, 20, 40, 60, 80, and 90 percent calcium carbonate. Figure 11 shows the ignition temperatures and the relative flammabilities of dust clouds of those mixtures. Increase in the proportion of carbonate resulted in rise in the ignition temperature and in reduction
of the relative flammability. Mixtures that contained 60 percent or more calcium carbonate could not be ignited by a high-voltage electrical induction spark, but in the furnace tests, where a hot surface acts as the igniting source, ignition and flame propagation could not be prevented until the mixture contained at least 90 percent calcium carbonate.
Dust clouds of the above mixture gave somewhat erratic results in taste on the determination of minimum igniting energy, minimum explosive concentration, and pressures developed by explosions. In some tests, mixtures with 10 and even 20 percent calcium carbonate were found to be almost as explosive as the pure starch itself. This is thought to be due to the fact that addition of small proportions of the carbonate reduces the stickiness of the moist starch and promotes the formation of dust clouds, which action masks the diluting effect of the inert carbonate.
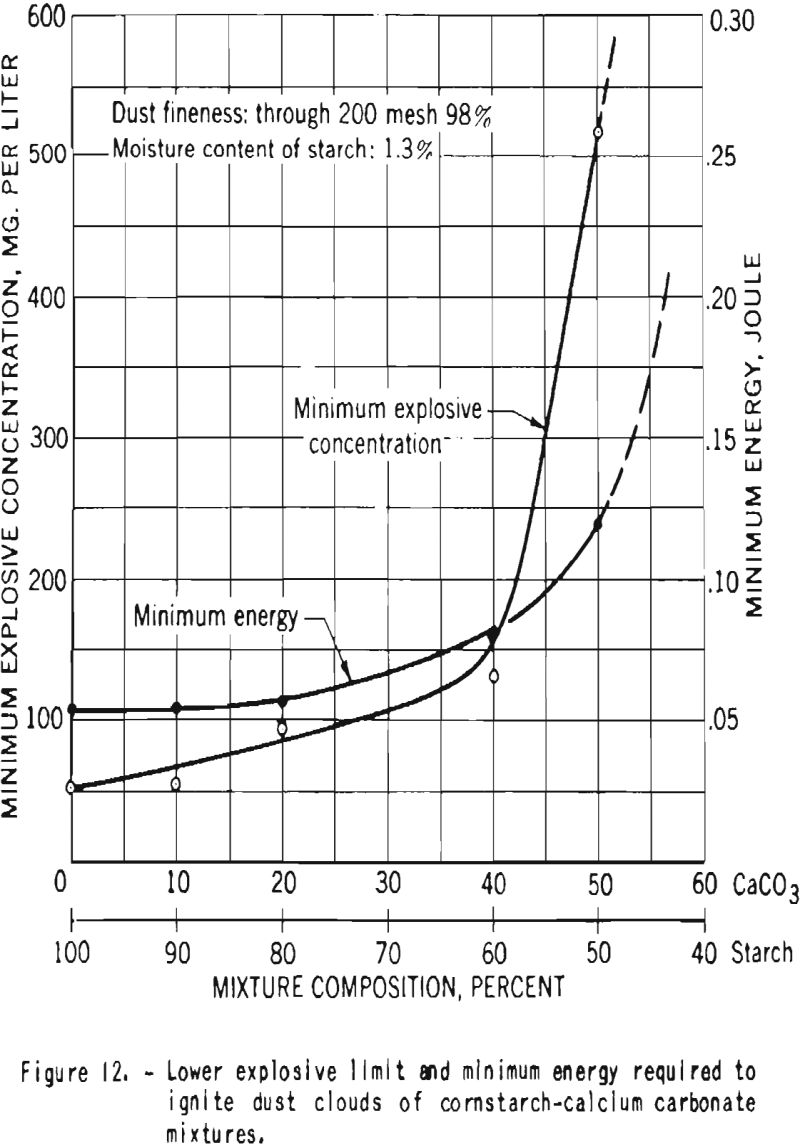
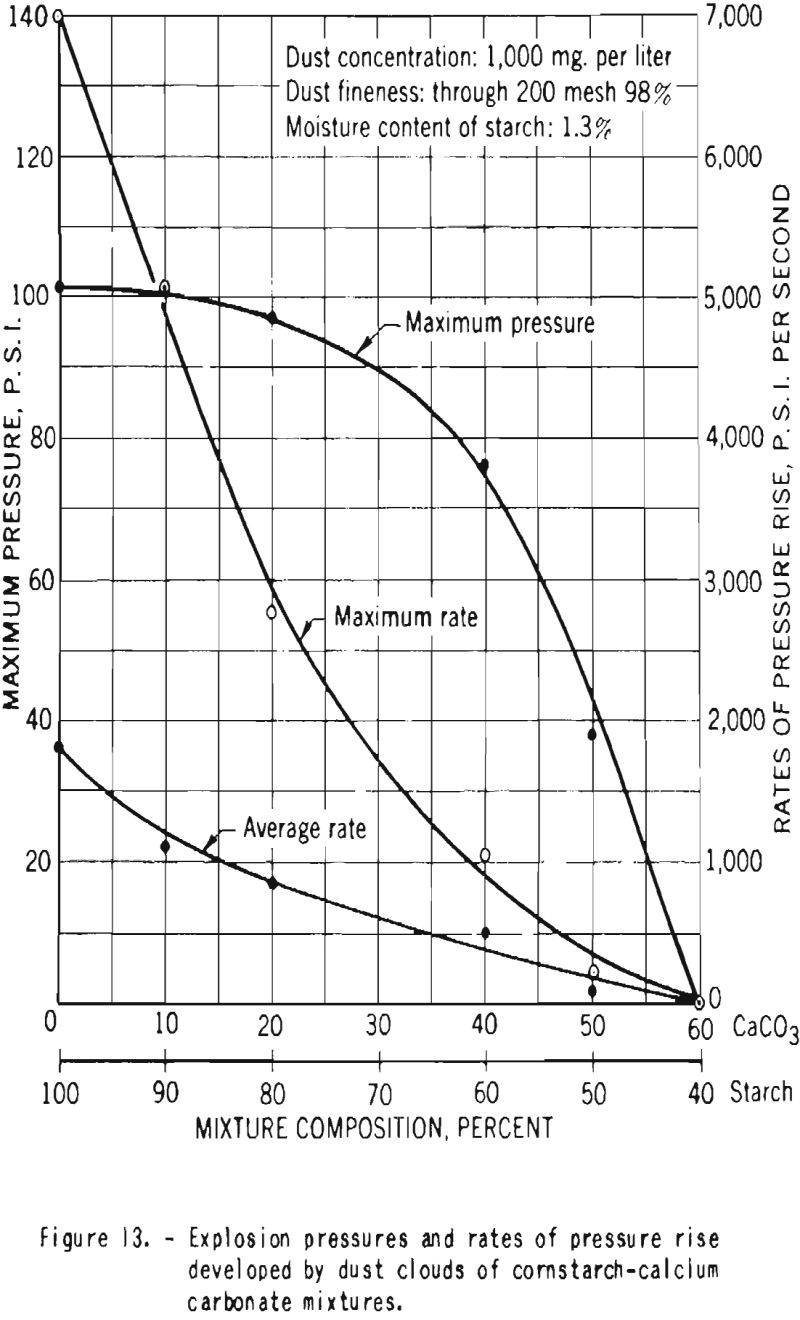
Additional experiments were performed with mixtures of dried starch containing 1.3 percent moisture and calcium carbonate. Figure 12 shows that the minimum explosive concentration as well as the minimum energy required for ignition of the dust clouds increased with increase in proportion of calcium carbonate. Figure 13 is a plot of the maximum pressures and the average and maximum ratios of pressure rise developed by explosions of dust clouds. Addition of 10 or 20 percent calcium carbonate to the starch resulted in great decrease in the rates of pressure rise but had little effect on the maximum explosion pressure, but further increase in the proportion of carbonate effected important reduction in the pressure. In these tests a high-voltage electrical spark was used as the initiating source, and no ignitions or explosions could be producod in mixtures that contained 60 percent or more calcium carbonate.
Release of Explosion Pressures
It is well known now that the most important safeguards against dust explosions are good housekeeping throughout a plant in order to reduce diceomination of duet, particularly to prevent the formation of dust clouds, and the elimination of all sources of ignition from dusty cross. In addition to these measures it is necessary to provide adequate vents to relieve the pressures in case an explosion should occur. These vents serve to limit the pressures developed within on enclosure and thereby reduce structural damage.
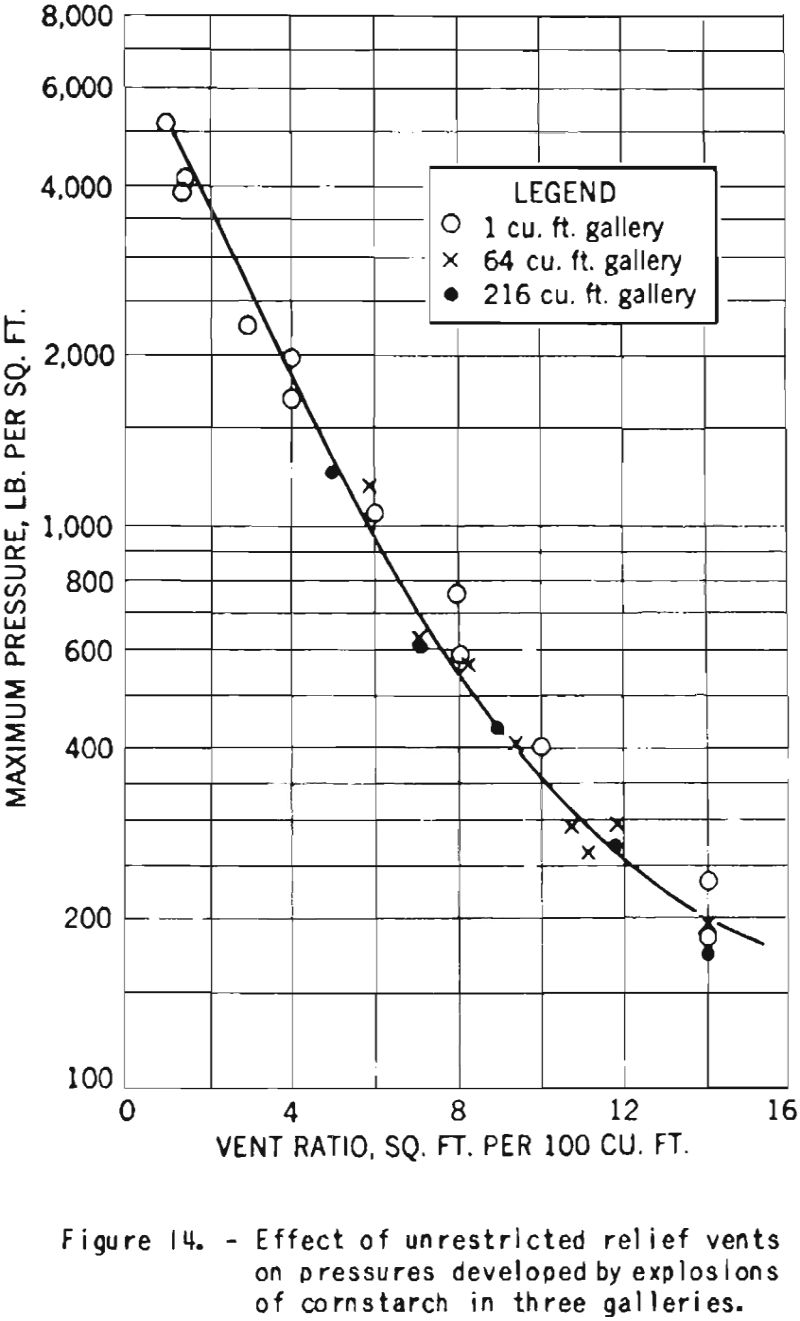
For the sake of completeness, there is included in this report a record of some earlier experiments on the venting of cornstarch explosions, shown in figure 14. The curve illustrates the great decrease in pressure with increase in vent area expressed as a ratio of area to volume of explosion space. The tests were made in cubical test chambers of three sizes. The proper sizes and the spacing of vents to be used in a particular structure depend on its strength and other design characteristics.
