Table of Contents
The improvement in leaching introduced by the Russell process has stimulated the development of processes for refining lixiviation-sulphides.
In the early days several processes for dealing with the sulphides were proposed, and some of them were tried more or less ; but the business finally settled down to sending the sulphides to the smelters, although this was known to be both troublesome and expensive. Mr. C. A. Stetefeldt introduced at the Marsac mill, Park City, Utah, an unpatented process, built up out of the general fund of information available. It consisted in matting the sulphides, grinding, roasting, grinding again, and dissolving the copper out in diluted sulphuric acid, then melting the silver and crystallizing the bluestone. It did not yield fine bullion, hence the bullion had to be refined as well as parted; besides, there was some loss. This process was thoroughly tried at the Marsac refinery and then a year’s run was made, the net result of which was that it did not prove sufficiently better than the sending of the sulphides to the smelters to warrant its substitution for that practice.
The Dewey-Walter Refining Company undertook the refining of the Daly sulphides in the Marsac refinery by the sulphuric acid process, upon which two United States patents have been issued to the writer. Naturally, difficulties were encountered in starting a new process, and most was taken up in getting it into smooth working order; but a run was started, in which all the regular sulphides produced by the Marsac mill in that year were refined, and thus complete statistics of operation by this method were obtained.
Broadly speaking, the process consists of six main operations:
- Boiling the sulphides with strong sulphuric acid in an iron pot;
- Dissolving out the sulphate of copper and silver in a lead- lined tank, leaving a residue containing the gold and lead of the sulphides, and also rich in silver;
- Precipitating the silver out of the filtered solution by copper plates;
- Sweetening, drying, pressing, and melting the cement- silver ;
- Treatment of the solutions after the removal of the silver to crystallize the sulphate of copper, and recover the excess of acid for re-use;
- Treatment of the gold-bearing residues.
The run of the Marsac leacher produced 116,519.5 pounds of regular sulphides which were treated by this process. For convenience they were divided into 25 lots, mostly from 4500 to 5500 pounds in weight. As reported by the assayer of the Daly Mining Company, these lots varied in composition as follows :
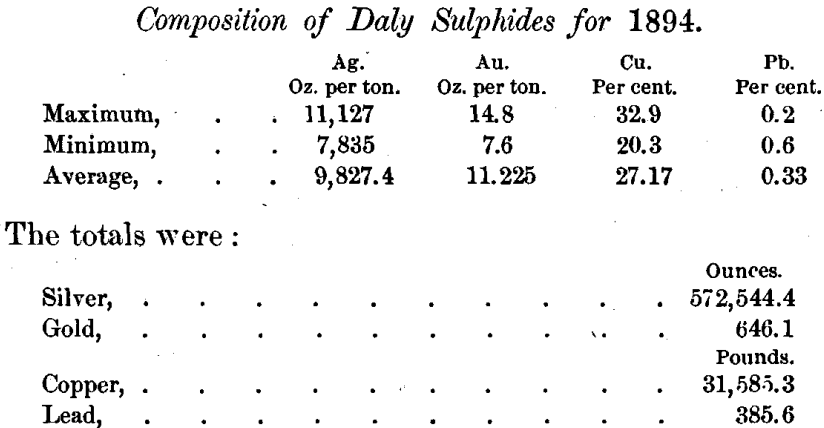
These determinations of lead are too low, the actual average for the year being about 2.5 per cent.
I have found the percentage of free sulphur in the Daly sulphides to vary considerably. It is generally rather low, and sometimes very low. Lots 8, 9, 10, 11 and 12 showed 3.3, 3, 2.72, 0.48 and 1.24 per cent, of free sulphur respectively. The total sulphur was found on one occasion to be 20.74 per cent.
Aside from the soluble salts remaining in the sulphides through imperfect washing in the filter-press, the drying causes oxidation, and soluble sulphates are produced. As received by the refinery, the sulphides contain several per cent, of soluble salts and small amounts of iron, lime, antimony, arsenic and other impurities.
Acid Leaching Plant
The plant required is simple and well known, and easily managed without specially skilled labor. It consists of two ordinary cast-iron pots, such as are used in parting bullion; a series of 21 lead-lined tanks for dissolving, filtering the solutions, precipitating the silver and filtering off and sweetening the cement-silver, together with crystallizers to recover the blue-stone and evaporators to concentrate the mother-liquors for re-use in the pot; a dryer and press for the cement-silver; a furnace for melting the bullion; 4 storage-tanks for acids, pumps for handling the liquids, and a pulverizer.
Boiling Pots
These are ordinary cast-iron pots. The large one for boiling sulphides is 47.5 inches in diameter and 3 feet deep inside, with a rim 3.5 inches wide, slanting slightly to the inside. It is 1 inch thick on the bottom and ½-inch on the sides. Each pot weighs about 1500 pounds. It rests on a cast-iron plate 5 feet 7¼ inches by 5 feet 6½ inches by ½-inch, with a 2-inch rim to fit over the brick setting. At first we obtained our pots as special castings from a New Jersey foundry, paying a corresponding price for them; but afterwards we obtained them at a smaller price from the foundry of Davis, Howe & Co., at Salt Lake City, who, moreover, take the old pots from us as scrap-iron at a good price. Their pots have done even better service than the New Jersey ones. The pots lasted from five to six weeks, but we have had better results. For 1 year, 7 pots were used, with an average life of seven weeks and five days, and treating an average product of 23,737 pounds each. The acid does not dissolve much iron during the boiling, but a net-work of cracks develops until the acid leaks through. With proper care there is little danger of loss of silver on account of the pastiness of the charge. About a day is usually lost in changing pots. Some of the pots were made 2 inches, thick on the bottom; but they were no better than the 1-inch pots. The average loss in weight per pot was 67 lbs., as shown by the difference in weight of 7 of the 94 pots when new and when sold as scrap-iron. The pot is set in brickwork over an ordinary grate, in which the local coal from Coalville, Utah, is burnt.
The top of the pot stands 14 feet from the ground-floor, so as to allow all the solutions to travel downwards from tank to tank by gravity. Over the top of the pot a spherical cast-iron hood, 49 inches in diameter and ½-inch thick, is placed, having a working door 18 inches by 9 inches on the side. At the top is an opening with a water-joint cast in, into which fits a 10- inch lead pipe, 6 feet long. This leads to a lead-lined stack going through the roof. This arrangement is to remove the fumes given off during the boiling. The draft can be increased by a steam-jet in the stack, as may be occasionally necessary.
The small pot for re-treating the residue is 2 feet 8 inches in diameter and 2 feet deep, resting on a plate 4 feet 3 inches square. This pot has a conical hood, made of sheet-lead over a frame-work of iron covered with lead. The large pot originally had a lead hood, but this was replaced by the cast-iron one, which has given better satisfaction. The foundation for the large pot is built up solid from the floor, but for the small pot 12-inch walls are run up to the fire-box level, leaving a chute for the discharge of the ashes.
The tools required at the pot are a hoe for stirring, a discharging-ladle, coal-scoop, short-handled shovel, broom, lead- bucket and a trough to carry the boiled charge to the dissolving-tank. This latter is a slight V-shaped trough of sheet-iron, which is placed in front of the pot when ready to discharge, and leads to the dissolving-tank.
Dissolving Tank
This is 4 by 8 by 2 feet, made of 2-inch rough boards dowelled, with the ends let into the sides ½-inch and bolted at the ends through buck-staves. All the tanks are made in the same way and thoroughly painted with asphalt. The first dissolving-tank was lined with 8-pound lead; but this was not heavy enough, and 12-pound lead is now used.
The contents of this tank must be heated by the introduction of steam. If this is done directly from a pipe attached to the lining, the vibrations set up will soon open a joint in the lead lining and cause a leak. To avoid this, a very simple arrangement was formerly used. It was a lead cone 15 inches long, fastened to the steam-pipe. It was perforated with 3/16-inch holes on the top and sides, and stood on feet about 2 inches from the bottom of the tank. The steam-pressure was gradually reduced by the cone and the solution heated up gradually and quietly. This arrangement worked well, but the cone soon wore out. At present a plain lead pipe, turned up at the end and entirely free from the lining, is used with satisfaction.
This tank is provided with two discharge-hose, one for each filter-tank, arranged to draw off the solution as free as possible from fine residue. Pieces of lead pipe 10 inches long, projecting through the end of the tank, are burned to the lead lining at the bottom, of the tank. A piece of rubber hose about 2 feet long is slipped over the inside end of the pipe. When drawing off’the solution, the open end of the hose is adjusted, by a lead string, just below the surface of the liquid, so as to draw off only the clearest portion. This arrangement is a great saving for the filters, which at best are soon enough choked up by the fine residue. This tank has a A-shaped lead-lined, counterpoised cover, with a triangular opening in the side for the introduction of the trough used to convey the charge from the boiling-pot to the tank.
Filters
The two filters are each 3 feet by 6 feet by 2 feet, lined with. 8-pound lead. Considerable difficulty wras experienced at first in constructing a suitable filter. The difficulty was to get a filter that would retain all the residue, giving a clear filtrate, and at the same time give a good rate of filtration. If the residue passed through, it reduced the fineness of the bullion and at the same time very small amounts of gold appeared in the fine silver and were practically lost. A satisfactory sand-filter which, by washing from below, could be easily freed from fine residue after becoming clogged, was finally constructed as follows: Sheet-lead was first bent so as to make slats 2 inches high by 1.5 inches wide. The bottom was open and the sides were notched at the bottom to allow free passage for the liquids. The slats are put about 2 inches apart on the bottom of the tank, and are 2 inches shorter than the width of the tank, leaving an inch at each end of the slats. On the slats is placed a plate of 8-pound lead perforated with 3/16- inch holes ½-inch from center to center, and then a thickness of cocoa matting. To make a tight joint between the slats and the side of the tank, a 6-inch strip of asbestos cloth is laid around and driven tightly against the sides by a tightly-fitting strip of hard wood. The asbestos laps over the cocoa matting about 2 inches. Next comes a second layer of cocoa matting and finally a second lead plate over all. On this foundation from 3 to 4 inches of clean quartz sand is spread and well-hammered down. A discharge-pipe runs out of the bottom of the filter at one end.
The hose from the dissolving-tank discharges into a perforated lead box on top of the sand, to prevent the stream from disturbing the sand. A steam-pipe for keeping the solution hot also discharges into this box. When new, this filter gives a large stream of clear filtrate. It must be carefully watched at all times. Its success may be shown by the fact, that notwithstanding the very fine nature of the residue and its liability to run through, 401 bars of bullion showed 999.5 and 45 bars 999, with an average of 999.4 fineness for the whole year.
The fine residue gradually chokes up the filter and in about 2 weeks the rate becomes so slow that it must be washed. This is done by running in water from the bottom and thoroughly stirring up the sand. The muddy water holding the residue in suspension is then pumped out into a small filter. This is repeated once or twice and the filter is smoothed off and hammered down, when it is ready for use again and will give a good rate of filtration.
Precipitating Tanks
The small precipitating-tank is 8 by 5 by 3 feet, lined with 8-pound lead. It is used for the first charge from the dissolving-tank, which contains more copper and is known as “ copper-solution.” The large precipitating-tank is 9 by 7 by 3 feet, lined with 8-pound lead and with an extra sheet of lead laid on the bottom. It was made large and of these dimensions to fit a space in the old refinery. It is used for the silver-solution. Each precipitating-tank is provided with an old syphon-pump for stirring the solution. A lead pipe reaches from the pump to the bottom of the tank, and when steam is turned on, it is allowed to suck in air, which mixes with the steam and stirs the solution.
Silver Filters
These tanks are 6 by 3 by 2 feet lined with lead and fitted with heavy asbestos cloth filters between lead plates, the whole resting on lead slats. One has an 1/8-inch iron cover in three sections and is used to sweeten the silver in, and the other is a general filter-tank through which solutions pass, after precipitation, to catch any fine silver in suspension. Each filter has a sump consisting of a tank of the same size lined with 8-pound lead.
The sheet-iron cover is convenient and gives good satisfaction, but it has been the occasion of an annoying incident. The bullion fell off from 999.5 to 999 ; and after a thorough investigation it was found that some unusually large charges of cement silver had been put into the filter, and probably the sag of the cover in the middle had touched the silver or the wash-water and reduced a little copper from the wash-water. At any rate the fineness rose again after stopping the practice of washing such large charges.
Evaporators
These are 8 by 4 by 2 feet lined with 8-pound lead. They have gridiron coils of ¾-inch lead steam-pipes connected together. Just enough steam is used to blow out the condensed water. They are housed in, the housing being provided with working-doors and a stack. They will evaporate away 75 per cent, of a 20° B. solution in 24 hours. A hose and launder deliver the concentrated solution to the crystallizers.
Crystallizers
These 7 tanks are 3 by 6 by 2 feet, lined with 6-pound lead and provided with false bottoms. Each one has a draining-board and 12 2- by 2-inch wooden strips across the top, from each of which hang 6 lead strips 3 inches wide. The finest crystals separate on the strips. The blue-stone crystals on the bottom are chopped out with iron bars, the false bottoms being employed to save the bottom when this chopping-out is done.
Residue Filters
Two small tanks are used for these; one for the residue from the dissolving-tank, and one for the muddy water from washing the large filters. They have no asbestos and only 1 inch of sand, but they have a very slow rate of filtering. A layer of residue on the bottom soon chokes them up so that it is almost impossible to get anything through; and most of the solution is usually syphoned off from the top after the residue has settled. Both filters discharge into the dissolving tank.
Acid Storage
The large storage-tank for 66° B. acid is 8 feet in diameter and 8 feet high, lined with 10-pound lead. It holds about 46,000 pounds of 66° sulphuric acid. The small tank is the old Roessler converter 4 feet in diameter and 6 feet high, and holds about 8000 pounds. Both these tanks have 2 stoneware cocks, one for every-day use, and the other a safety on the end of a length of lead pipe, ordinarily hung up at the top of the tank.
A section of 15-inch wrought-iron pump-column, holding about 1500 pounds of acid, is used as a monte jus, to elevate the acid by air-pressure, and two sections are used to store a supply of acid above the level of the pots for feeding them.
The evaporated acid is stored in a tank 5 feet 6 inches by 3 feet by 2 feet below, and in two round tanks, 3 feet 6 inches in diameter and 5 feet high, above, convenient to the boiling-pot.
Silver Dryer
After sweetening, the cement-silver is removed from the filter and put into sheet-iron drying-pans 2 feet 6 inches by 1 foot 2 inches by 3¼ inches, made of No. 10 iron. The bottom and sides are in one piece and the ends are riveted in. The dryer consists of a frame-work of angle-irons enclosed in brick-work. It holds 6 tiers of pans, 4 in each tier, or 24 pans in all. Under each one of the bottom 3 tiers of pans are arranged 5 4-inch steam-pipes joined together at the ends by cast-iron heads. When the dryer is full the pans are arranged so that the hot air zigzags below and above each row of pans from bottom to top, and then passes out through a small stack.
Hydraulic Press
This is of Watson and Stillman’s make. It is set at 1000 pounds and pure glycerine is used for the fluid. The mold is 6 inches in diameter and 4 inches high. The pressed cakes are about 1¼ inches thick and weigh 60 to 70 ounces.
Melting Furnace
This is of the ordinary type for 2 No. 50 black-lead crucibles and provided with 3 dust-chambers.
Pulverizer
A small Bruckner ball-mill, 800 mm. in diameter and 500 mm. in width, with 40-mesh screens, is used for pulverizing the slag, etc.
Pumps
Two No. 2 Sorting hard-lead syphon-pumps with platinum nozzles are used to handle the solutions. Pumps made of all hard lead from the Hanover works have given better satisfaction than those enclosed in an iron shell.
Installation
The plant was installed in the building erected for the Stetefeldt process of refining, and as much as possible of the old plant was utilized in the installation. Necessarily, therefore, the arrangements are not as convenient and satisfactory as they might be. The main building is 52 by 42 feet with an annex 52 by 25 feet in size; but most of the annex is taken up for space in handling the sulphides before they are turned over to the refinery, and the silver-dryer stands outside the building. Recently a new melting-room with hearths has been erected.
Process
The process consists in boiling the sulphides in strong sulphuric acid to convert the sulphides into sulphates. The sulphate of silver is soluble in strong sulphuric acid, but the anhydrous sulphate of copper is practically insoluble. Owing to the large percentage of copper in the sulphides a large quantity of insoluble sulphate is produced, and this is one of the most serious difficulties of the process. After boiling, the charge is removed to the dissolving-tank, into which are put pure water, wash-water and weak solutions. Here the copper sulphate goes into the solution along with the sulphate of silver. The solution is filtered into the precipitating-tanks, where the silver is precipitated by metallic copper, after which, when strong enough, the solutions go to the crystallizers to recover the bluestone. Periodically the cement-silver is removed to the filter, sweetened, dried, pressed and melted. The mother-liquors are concentrated and crystallized, and the recovered acid is finally sent back to the pot. The residue in the dissolving-tank is taken out, washed somewhat and re-boiled, to recover as much as possible of the silver that it contains.
Practical Operations
The first charge of the sulphides was put into the pot and the first charge of the later sulphides was started, so that the run was a few days over a year. A part of this time was taken up in the annual clean-up. A charge of 975 pounds is put into the pot in the morning with about 1000 pounds of acid (66°) and thoroughly mixed and the charge heated. At first the reaction is rather violent. SO2 is copiously evolved and the fumes carry considerable S, which gives them a yellowish color. At this stage the steam-jet may be required to increase the draft. After a while the reaction settles down and the normal charge boils quietly until near the end. As soon as the charge gets stiff, more acid, about 100 pounds, is added, until about 3000 pounds have been used. Toward the end, evaporated acid is used. The strong acid is first drawn from the overhead receivers into a cast-iron pan, an old clean-up pan from the amalgamating-mill, holding about 180 pounds, through a stone-ware cock. It is then run into the pot through a rubber hose.
As the boiling goes forward, the anhydrous sulphate of copper is formed in large quantities, which separate, forming granular masses. This necessitates frequent stirring of the charge; and this, in turn, is severe on the pots.
The progress of the operation can be watched by taking out a small sample of the charge, treating with water and adding HCl to the solution; but this is not necessary after getting familiar with the process, since the color changes from black to brown or gray. About 90 per cent, of the total acid used is added before the charge begins to show soluble silver salts. Then the charge foams violently and must be constantly stirred, while the fire must be lowered. In about an hour the foaming is over and the charge is finished, This usually occurs in the afternoon of the day after starting.
The boiling is done in day-shifts only. The charge could now be removed to the dissolving-tank, but it is too thick, from the large amount of separated sulphate of copper, to be syphoned out; so it would have to be dipped out with a ladle. As it is now very hot and giving off SO2 freely, this would be a disagreeable operation.
On the second morning, therefore, the charge is warmed up, generally with the addition of some acid, until it is sufficiently fluid, when it is ladled out into the sloping trough, which delivers it to the dissolving-tank. The pot is then started on a new charge. Although a charge could be turned in less than 2 days, this makes a very convenient and generally economical arrangement.
When starting the charge and when finishing it, the pot requires much of the potman’s time; but at other times it does not call for much attention. The potman attends also to the dissolving- and the filtering-tanks, and to the precipitation of the silver. The ladle used in emptying the pot is a wrought- iron scoop and holds about half a gallon.
The dissolving-tank is filled with cold water to within 6 or 8 inches of the top and tightly covered, since the introduction of the charge generates much heat. After the charge is in, the cover is raised and the solution is stirred with a wooden paddle and boiled with steam, after which it is settled about half an hour and drawn into the filters. The first tankful of solution contains most of the copper, and is run into the small precipitating-tank and kept separate from the rest of the solution. After the precipitation of the silver it contains, it is run directly to the evaporators, and brought up to 35° B. and then crystallized.
The charge now resembles thick white mud and is washed from 8 to 10 times with weak acid solution, to remove the silver, yielding solutions about 20° B. in strength.
After washing, the residue is thrown into a filter. This residue varies very much, running from 5,000 to 19,000 ounces of silver per ton and 50 to 100 ounces of gold, the balance being mainly sulphate of lead. Some of the silver is present as sulphate, but there is also considerable metallic silver.
The solution has a reducing action, and immediately a separation of metallic silver begins in the dissolving-tank, often with the formation of beautiful growths upon the surface of the liquid. This reaction continues in the filters. By reason of it, metallic silver is found in the first residue, and some 10,000 ounces may accumulate in the filters during the year’s run. In the annual clean-up, the sand is removed from the filters and boiled in the pot with strong acid to recover the silver. A small amount of silver and some gold remain in the sand.
From the filters the silver-bearing solution goes to the precipitating-tanks, where the silver is precipitated by copper, cathode-plates from an electrolytic refinery being used. The plates are stood up against the sides as close as possible. The precipitated silver falls to the bottom and is scraped away from the plates every few days with a wooden shovel. The copper solutions require along time to precipitate, sometimes 18 hours, but ordinary solution is precipitated in 4 to 5 hours. During the precipitation the solution is stirred by air and heated by steam.
When the precipitating-tank is cold and the hot solution of silver sulphate runs in, there may be a separation of silver sulphate, which may go into solution again as the solution is heated up, but some of it may also remain in the cement-silver, and be removed in washing the silver, in which case the wash-water must be treated with copper. This is done in the wash-solution storage-tank.
When about 20,000 ounces of cement-silver have accumulated in the precipitating-tanks, it is shoveled out into the sweetening- tank with a wooden shovel, washed with hot water and then with acidulated hot water until the ammonia-test shows no copper. This takes about 15 hours. The wash-water runs through a guard-tank containing scrap-iron, and then to waste. The sweetened silver is put into iron pans and dried about 24 hours in the steam-dryer, pressed into cakes, dried again arid melted. A small copper shovel and an ordinary wisp-broom are used in handling the cement-silver at the press.
The melting was done in graphite crucibles, holding about 2400 ounces or 2 bars each, coke and charcoal being used for fuel. The crucible was filled with cakes and a little borax was added. As the cakes melted down, more were added with a pair of tongs, until the crucible was nearly full of molten silver. Niter was then added, and sometimes a little borax, and the crucible was stirred with an iron rod, after which the slag was removed with an iron skimmer. This was repeated until the surface became clear and bright. After three or four additions of niter, the metal begins to boil. The chief impurity in the cement-silver is the iron rust from the pans, which is easily removed in the slag. About 1.4 pounds each of borax and niter are used for each crucible-charge. The cost of melting was about 0.1 cent per gross ounce.
When the slag has all been carefully removed, the metal is stirred and sampled. The crucible is then hoisted out of the furnace and the metal is cast. The melted silver is poured into heated and greased, light cast-steel molds. After pouring, a little sugar is thrown on the liquid silver and the mold is covered by a tight-fitting cast-iron cover. This gives a very smooth surface to the bar. When cool the bars are hammered up and marked. The average fineness for 1894 was 999.4 silver, with no gold. As already stated, 446 bars were produced, of which 401 were 999.5 fine and 45 were 999 fine. The ashes and slag were ground and the buttons of silver separated, after which they were sent to the smelter.
The dissolving-solution is sent back to the dissolving-tank, after the precipitation of the silver, and is thus used again and again. It gradually increases in bulk, from condensed steam, and the copper contents increase. When it reaches 20° to 25° B. it is filtered and run into the evaporators and brought up to 35° to 37° B. It is then run into the crystallizers and left 2 days, if there is time, which separates most of the blue-stone.
The solution then goes back to the evaporators, and is brought up to about 42° B., and crystallized again. This crop of crystals contains a great deal of sulphate of iron. After the removal of the solution the crystallizer is filled with cold water, which dissolves most of the iron with but little of the blue-stone. This solution is run to waste through the guard-tank and the blue-stone remaining is shoveled out. This removes most of the iron from the blue-stone without the loss of much blue-stone. This product is not as good as the rest, the crystals being very small, but it answers as well in preparing “ extra solution ” in the leacher.
The main solution is again evaporated to 50° to 52° B., and run into the crystallizer, where it is allowed to stand several days, to separate as much as possible of the iron it contains. It is then pumped to the storage-tanks above, and used in the pots. Considerable iron is deposited in the evaporators during the second and third evaporations. Periodically they are washed out clean with water and the solution is run to waste through the guard-tank.
After boiling five charges of sulphides about 750 pounds of wet residue are obtained. This is put into the pot and boiled with a little more than its own weight of acid, after which it is washed and the final residue dried. This residue is very complex in composition, although it is mainly sulphates of lead and silver.
Various attempts were made to analyze this material so as to show how the different substances were combined and distributed. Finally two samples were put through a course of analysis, which, it was thought, would yield interesting and valuable results. The results obtained are interesting and valuable too, but they cannot be considered as harmonious or even satisfactory. I give the actual results obtained. I have spent considerable effort in trying to figure them out to a satisfactory conclusion, but have not been able to do so.
Two samples were examined. They were first treated with ammonia solution and filtered. The filtrate was neutralized with acetic acid, giving a precipitate of AgCl, 3.40 per cent, in one and 1.55 per cent, in the other. HCl was then added to the second filtrate, giving 6.14 and 1.60 AgCl respectively, equal to 4.62 and 1.20 per cent, of Ag, or 6.67 and 1.74 per cent, of Ag2SO4. BaCl was then added to the third filtrate, giving 16.89 and 16.52 per cent. H2SO4, of which 2.09 and 0.54 per cent, corresponded to the amount of Ag2SO4 found, leaving 14.80 and 15.98 per cent, possibly existing as free acid, or in some other combination. There is a certain quantity of free acid present, but it is hardly possible that all this acid could be free, since the samples are dry powders. A separate attempt was made to determine the amount of free acid present; but the difficulties in the way rendered this somewhat unsatisfactory. The best determinations indicate that one sample contained about 2.27 per cent., and the other 0.59 per cent, of free H2SO4.
The filtrate from the BaSO4 was treated with H2SO4, and 0.48 and 0.60 per cent, of PbSO4 were obtained. The final filtrate was treated for copper, and showed 0.46 and 0.16 per cent.
The residue was next treated with HNaCO3 to decompose PbSO4. Silver was not found in this solution. 22.95 and 18.10 per cent, of H2SO4 were obtained. The determinations of the lead showed that 19.82 and 17.62 per cent, of this acid were combined with lead to form normal PbSO4, leaving 3.13 and 0.48 per cent, in some other combination. In the filtrate from the BaSO4, 0.18 and 0.57 per cent, of PbSO4 were found.
The residue was next treated with acetic acid, and the following results were obtained:
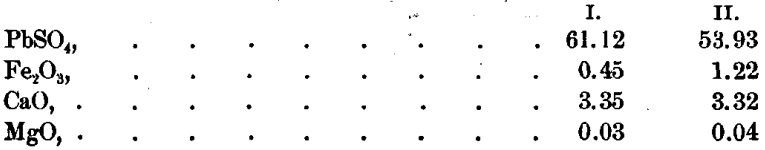
Ag was not found in this solution.
The residue was next treated with nitric acid, and HCl added to the filtrate, giving 7.96 and 19.69 per cent, of AgCl, equal to 5.99 and 14.82 per cent, of metallic Ag. Most of this Ag was probably present in the metallic state, but there was also a little unaltered sulphide of silver present, and possibly other combinations of silver.
This solution also gave the following figures:

The residue was again treated with ammonia, and yielded 0.40 and 0.30 per cent. of AgCl, and 0.09 and 0.02per cent. of PbSO4. The final insoluble residue after all this treatment was 5.78 and 5.26 per cent.
Separate portions were treated with Cl in alkaline solution, and yielded the equivalents of 39.32 and 34.08 per cent. of H2SO4.
The following table gathers up the results. The sulphuric acid is stated as H2SO4, but only a small portion was actually present as such :
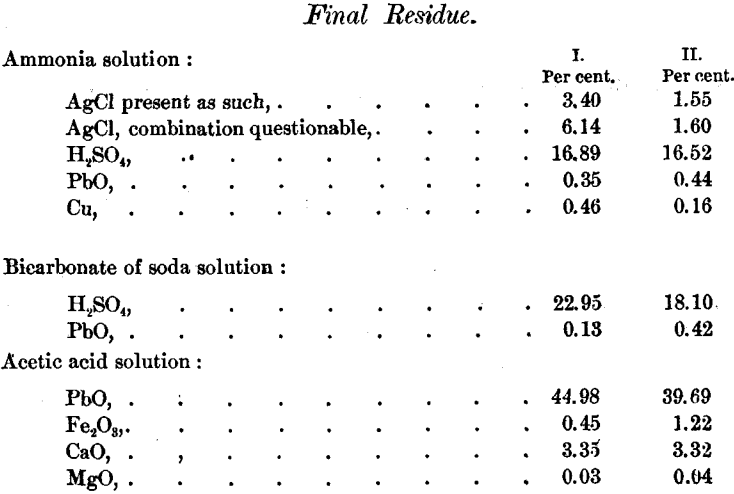

The final residue and the earthy constituents undoubtedly came from the sand of the filter gathered up in handling the material in the refinery.
This residue was shipped away to the smelters for treatment, but now it is being melted on a hearth at the refinery.
Supplies
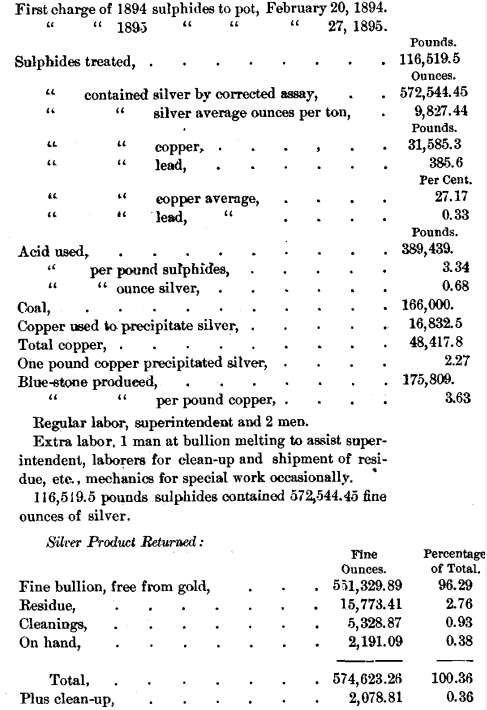
Sulphuric acid of 66° B. is purchased at Colo., and shipped to the refinery in iron tank-cars, holding from 40,000 to 50,000 pounds. It is drawn or syphoned out of the cars into a lead-lined iron tank on a truck and hauled to the refinery, where it is discharged into the storage-tanks by means of compressed air. It is necessary to have this truck-tank lined with lead, for the reason that, on standing, the small amount of acid necessarily left in the tank will absorb moisture and become dilute enough to attack iron. The handling of the acid requires some care, but presents no difficulty. It would be better if the acid could be blown by compressed air directly from the tank-cars to the storage-tank; and this arrangement may be made. In the run 889,439 pounds of 66° acid were used, an average of 3.34 pounds per pound of sulphides treated, or 0.68 pound per ounce of silver.
The particular form of copper used is of no consequence, provided it does not contain impurities that will reduce the fineness of the silver. At first we used cast-copper-plates, but afterwards used cathode-plates from an electrolytic copper-refinery. They were used direct without any preparation. When a pig-copper sufficiently free from gold can be had, it would be cheaper, especially if it carried silver, which would be recovered in the process.
In the run 16,832.5 pounds of copper were used. One pound of copper precipitated 2.27 pounds of silver, or 33.1 Troy ounces. This consumption of copper is larger than theory requires, but probably some copper is oxidized by the air used in stirring the solution.
The local coal from the neighboring Weber field is used. Other and more expensive kinds have been tried, but do not seem to present any advantages. In the run 166,000 pounds were used for all purposes.
Labor
For the ordinary operation of the process a superintendent and two men were required. Besides the general supervision of the work, the superintendent did the melting of the bullion about once in twelve to fourteen days, and required the assistance of an extra man. One laborer had charge of boiling the sulphides in the pot and the dissolving, filtering and precipitation of the silver. The other man had charge of the blue-stone and pressing the cement-silver. He also did the lead-burning, not only for ordinary repairs, but in the construction of the plant. Occasionally extra labor was required, particularly in shipping residue and making the annual clean-up. Mechanics were also required for special work at times.
Returns
It is well known that there is a loss in determining the precious metals, particularly silver, by fire assay, arising from absorption by the cupel and slag. In the case of ordinary ores the amount of this loss per ton of ore is generally small, although the percentage of the total silver in the ore be large. In the case of rich materials, however, the percentage loss on the total silver is low, but the actual quantity per ton becomes considerable, and when the rich material carries copper, the loss of silver per ton becomes quite serious.
In our business transactions the sulphides are always settled for upon an assay corrected for slag- and cupel-absorption as follows:
“ Weigh out one-twentieth (1/20) of an assay-ton of sulphides, 55 grams of granulated test-lead and 2 to 3 grams of fused borax.
One-half of the lead is put in the bottom of the scorifier and hollowed out; the sulphides are put into the hollow and the rest of the lead poured over them; the borax is then placed on top. The assay is then conducted in the usual way. The slag and cupel shall be ground up and assayed, and the results added to the main assay.”
This assay shows from 100 to 200, or even more, ounces per ton more than the ordinary uncorrected assay shows.
Even on the corrected assay the actual amount of silver returned by the refinery on the year’s work was 2,078.81 ounces more than the assays called for. Mr. Russell, who has had large experience in this department of assaying, has declared to the writer that, in his opinion, the very best assay of Russell sulphides that can be made, shows still about ¾ of 1 per cent, below the actual amount of silver present. Of course, there must necessarily be some loss in our practical operations, but these returns show that this loss is less than the difference between the corrected assay and the amount of silver actually present. It is regarded as an extraordinary showing for a chemical process on the large scale to recover more than the best possible assay calls for.
The sulphides treated, 116,519.5 pounds, contained 572,544.45 ounces of silver; and the silver returned was divided as follows:
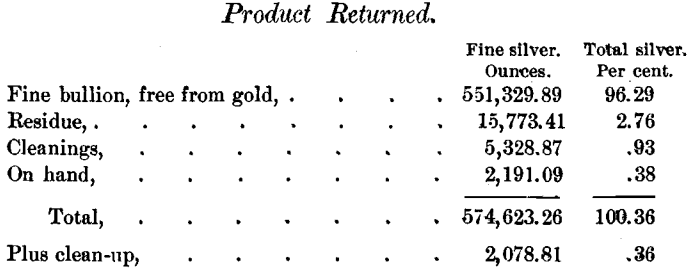
All weights of sulphides and products, excepting one case covering less than 200 ounces, and all the assays are the originals made by the Daly Mining Company. On selling the silver the reclamations by the buyers amounted to only 130.6 ounces for the whole year.
As to the recovery of the gold, I cannot see any reason why it should not equal the silver recovery, but the figures upon this point are not satisfactory. The actual return of gold for the year was 606.9 ounces, the original assays of the Daly Mining Company called for 654.8 ounces, but their re-assay on some of the samples reduced this to 646.1. This left an apparent shortage in the returns of 39.2 ounces. The same samples were assayed by Mr. Charles Earl, under my direction, and while the silver results showed a satisfactory agreement with the Daly assays, yet his gold determination called for only 602.9 ounces, showing a plus clean-up on the year’s work of 4 ounces. After the close of the year’s business, a general sample was prepared by taking proportionate weights of each of the check samples of the 25 lots, and the Daly Company’s assay of this sample called for 605.9 ounces, and showed a plus clean-up of 1 ounce. Mr. Earl is no longer with me, so I cannot give his figures on this sample.
There are special difficulties in determining such small quantities of gold in the presence of so much silver. The Daly Company’s assayer assayed the same samples of 3 lots of sulphides at three different times with the following results :
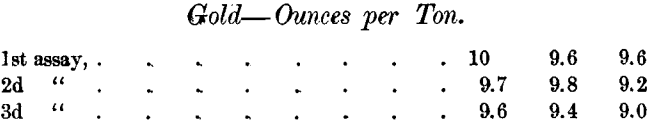
Mr. Earl found in the same samples 8.8, 9 and 9 ounces respectively.
These assays came on a run of about 24,700 pounds of sulphides, and the difference between the Daly’s first and last assays changed a minus clean-up to a plus clean-up.
Again, the Daly Company assayed a sample of sulphides at two different times, and Mr. Earl assayed the same sample at two different times, with the following results:
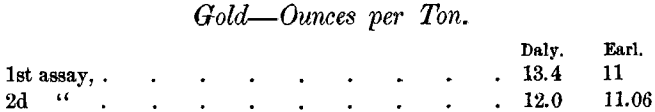
The difficulty of determining the gold is not confined to the sulphides. The Daly Company’s assays of 2 samples of gold bearing residue in triplicate were as follows:
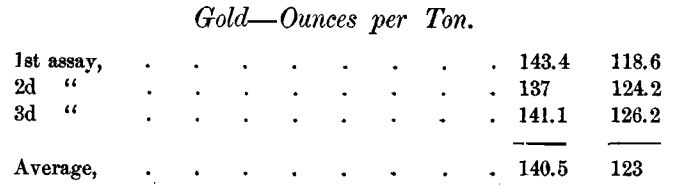
Another average on the first sample was 138.1. In one shipment of this residue the smelter paid for 0.48 ounce of gold more than the Daly Company’s assay called for.
It has been suggested that the silver bars were not entirely free from gold. While this was the case in the early days of the process, yet the assayer did not report any gold in any bars of silver shipped. With his previous experience in this matter, and with the care used in looking for gold, it is hardly possible that any considerable quantities of gold would have slipped out in the silver. A few ounces might possibly have escaped, but hardly 39 ounces.
The conditions of the process are such that I do not see how we could gain so much on the silver and lose on the gold; so that I am satisfied that the process practically recovers all the gold that goes into the operations, although the assays may not always show this.
The blue-stone product amounted to 175,809 pounds, or 3.63 pounds per pound of total copper going to the refinery. This copper includes the copper in the sulphides and the copper used to precipitate the silver. The return was somewhat below theory, but an unknown quantity of copper was recovered in the guard-tank, through which all the solution is passed before going to waste.
No particular care is taken to prepare fine large crystals of blue-stone ; and it is not necessary to purify the solutions from iron except as above described. Most of the blue-stone produced goes to the leacher, and the size of the crystals is of no moment whatever, while the small amount of iron present does no harm. The best grade showed 0.34 per cent., the medium 0.69, and the worst, of which only a small quantity was produced, 3.89 per cent, of protoxide of iron.
About 125,000 pounds of blue-stone were used by the leacher in preparing extra-solution, leaving about 50,000 pounds to be sold to outside parties.
Summary of Statistics for the Year of
Boiling Sulphides in Strong Sulphuric Acid
by the Dewey- Walter Process.
The advantages of this process are:
- It recovers a phenomenal percentage of the silver.
- It is an entirely liquid process from the beginning to the end, so that there is no loss from handling dry products.
- There is no roasting to cause loss.
- A large percentage of the silver is recovered as very fine bars, ready to enter the market.
- The process is so simple and so easily carried out, and the plant is so small and inexpensive, that it can be installed at individual leaching-works.
- Finally, the cost of operation is small; in fact, the value of the blue-stone recovered returns a large proportion of the operating-expenses.
