Minor-element interactions in equilibrated samples of copper matte and silica-saturated iron silicate (fayalite) slag have been studied by the Bureau of Mines to gain basic and fundamental scientific information that will support the mineral industry’s effort to improve productivity, increase energy efficiency, and reduce undesirable environmental impacts. This work was carried out as an adjunct to a previous Bureau investigation on the behavior of minor elements in copper matte smelting operations The previous study focused on the combined disposition of five minor elements (As, Sb, Bi, Se, and Te) present simultaneously in the matte-slag system, whereas the present study reports the individual behavior of the same five minor elements present singly in the same matte-slag system. Any difference in the distribution or behavior between the multiple-element and the single-element study indicates the existence of a minor-element interaction.
Minor-element behavior has been studied extensively in the slag-matte-metal systems of various copper extraction processes. Mackey presents an exhaustive review of the existing literature in this area. Included are theoretical predictions of minor-element behavior made on the basis of thermodynamic data. However, in many cases, the necessary data are not available, and therefore, laboratory studies of the equilibria have been performed.
While minor-element behavior in copper smelting systems has been of considerable interest, the interaction among the minor elements in metal-matte-slag systems has attracted limited attention. Minor-element interaction parameters are limited to simple metallic ternary systems.
In the area of copper smelting, one study focused on the quantitative determination of the interaction coefficients in the systems Cu-Pb-Bi and Cu-Pb-Sn. The present work investigates the possible existence of minor-element interactions in the copper matte-slag system, but does not present interaction coefficients. Based on results of the previous work, there is reason to suspect minor-element interactions because some elements saturated the matte-slag system. Bismuth and tellurium in particular saturated the system at very low levels, and prills of elemental Bi and clusters of Cu2Te were evident in the matte through electron microprobe analysis.
The behavior of minor elements in other copper smelting studies provides more reason to suspect that distribution co-efficients and solubilities may indeed vary according to the presence of other minor elements. A concept of molecular dissolution of Se and Te in the slag has been developed on the basis of thermodynamic properties of slag, and the observed solubility of Se and Te was explained in terms of the stability of molecular clusters of FeSe and FeTe in the slag. Therefore, it is possible that interactions among Se, Te, and other minor elements could affect the solubility and distribution between matte and slag.
Minor element interactions also were studied in the metallic copper-slag system. In this work, the combined concentrations of the minor elements (Pb, Bi, As, and Sb) were kept below 0.5 to 0.6 wt pct, or in the dilute solution region. Interaction among the minor elements was shown to be negligible. Previous Bureau work on copper matte-slag systems with all five elements present simultaneously typically had combined concentrations of As, Sb, Bi, Se, and Te between 2 and 3 wt pct of the matte. The combined concentration of minor elements in the slag was much lower than in the matte, ranging from 0.1 to 0.2 wt pct. This and the fact that the copper matte- slag system became saturated with two minor elements, Bi and Te, provide a reasonable cause to anticipate an inter- element effect in this system.
Experimental Procedures
The current investigation is concerned with minor-element behavior in equilibrated samples of copper matte and silica-saturated fayalite slag. Primarily of interest is the minor-element distribution or solubility when each element is present individually compared with the distribution or solubility when all five elements (As, Sb, Bi, Se, and Te) are added simultaneously. Copper and iron sulfides, fayalite slag, slag additives CaO, Al2O3, and MgO, and one of the accessory elements were equilibrated at 1,250° C under controlled gas atmospheres comprising CO, CO2, and 10 pct SO2. For each of the five elements, there was one equilibration with pure fayalite slag and nine more with three different levels of the three slag additives CaO, Al2O3, and MgO. The procedure, apparatus, experimental conditions, and analytical techniques have been described previously. For matte-slag systems not saturated with impurity element X, the distribution coefficient was defined as
Lx = wt pct x in matte/wt pct x in slag…………………………………………….(1)
Results and Discussion
Although detailed analyses exist of the interaction coefficients of minor ele-ments in liquid copper, there is no work on interaction parameters in copper matte or iron silicate slags. In most cases, the slag is assumed to be a dilute solution, and the minor elements dissolved in slag are assumed to obey Henry’s law. No study addresses interaction parameters in copper mattes, but one work focuses on converter operations and metallic copper saturated with Cu2S.
In that study, the Interaction parameter, ε S M, between sulfur and M, a chosen minor element, was assumed to be zero for M = As, Bi, Sb, Se, and Te. In another work on minor elements in the metallic copper-slag system, the interaction between Pb and Bi in liquid Cu was analyzed in detail, and the effect of Bi on the activity of Pb in liquid Cu was found to be negligible with 0.1 wt pct Pb and 0.5 wt pct Bi in the copper melt. The elements Bi, Sb, and As (all group V-A elements) were assumed to behave similarly; thus, Sb and As also would have no discernible effect on the activity of Pb, apb, in the copper melt.
The assumption of Henry’s law behavior for the minor elements should be substantiated by such a rigorous analysis because a dilute solution may not truly exist in a given system. The copper matte-slag system studied in the previous work was saturated with Bi and Te, which formed additional phases in the matte. It is not reasonable to maintain that in this saturated system either matte or slag is a dilute solution, with respect to Bi and Te, or that Bi and Te behave according to Henry’s law. The prediction of Henry’s law behavior for the remaining elements, As, Sb, and Se, is not possible without direct experimental evidence.
However, since Sb, Bi, and As are all group V-A elements and Se and Te are group VI-A, it is reasonable to assume, as in the previously mentioned study, that elements from within the same group would have a negative effect on each other with respect to solubility in matte or slag. Since the mechanisms of dissolution would likely be the same for the elements within the same group, the behavior of these elements could be characterized as a competition for available sites. In fact, this is most likely the only type of interaction observed in this work, as supported by experimental evidence.
Arsenic
The behavior of As as the only minor element in copper matte-slag systems at equilibrium was similar to its behavior when significant amounts of other minor elements were present. Distribution coefficients for As as the only minor element in the matte-slag system are shown as points in figure 1. These points can be compared with the regression line for the data published in the previous work, when As was present in the system with the other minor elements Sb, Bi, Se, and Te. In general, LAs was equivalent to or slightly larger than those values obtained in the previous work. Most LAs values were between 1 and 5, with a few exceptions. According to these data, LAs is independent of matte grade.
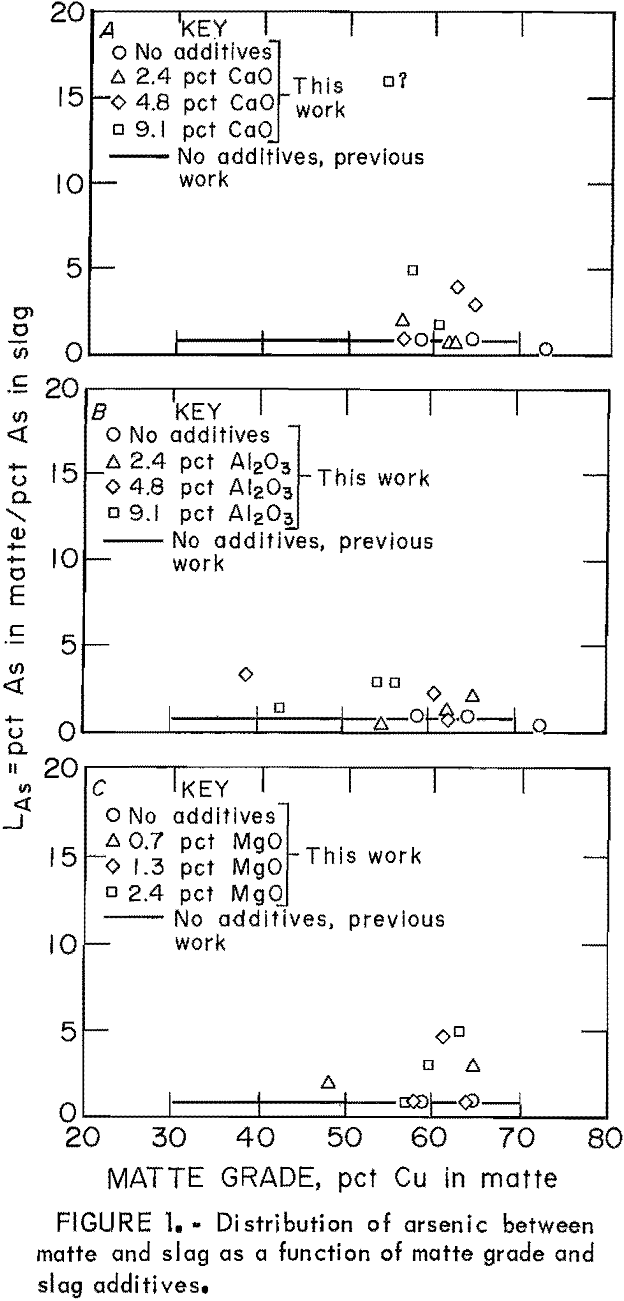
Levels of As were very low in mattes and slags from samples to which little or no CaO and Al2O3 was added. At concentrations of 0.0001 to 0.001 wt pct As, it is suspected that both slag and matte are dilute solutions obeying Henry’s law with respect to As, Experimental conditions in which the interelement effect would be expected include those tests with slags that had high levels of CaO or Al2O3. In these samples, As retention was much greater than it was with no slag additive, and concentrations of As in mattes and slags approached 0.1 wt pct. However, the present values for LAs do not differ significantly enough from the previous work to suggest any interelement effect.
Antimony
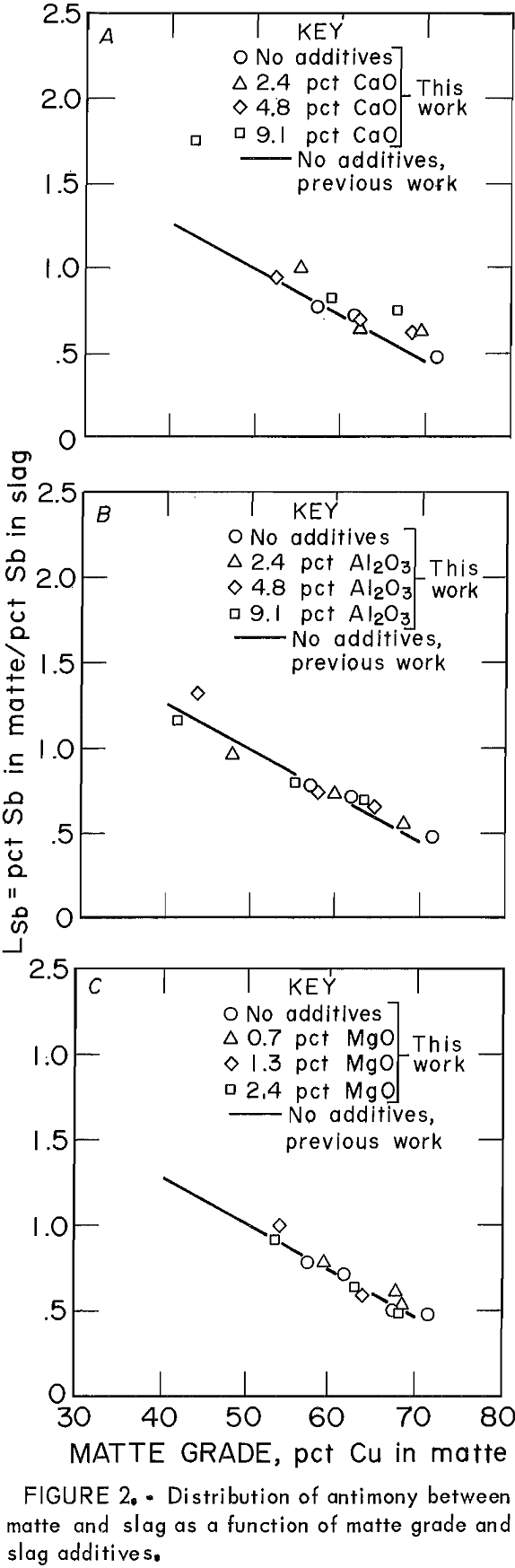
The behavior of Sb as the only minor element in copper matte-slag systems at equilibrium was similar to its behavior when significant amounts of other minor elements were present (fig, 2), Distribution coefficients for the present work are shown as points, which can be compared with the regression line for the data obtained in the previous work with As, Bi, Se, and Te also present in the system. When Sb was present with other minor elements, the effect of slag additives CaO, Al2O3, and MgO was to lower the Lsb slightly from the baseline data of no additives. When Sb was present alone, however, the effect of slag additives was not noticeable.
The distribution of Sb was essentially identical whether or not there was a significant amount of other minor elements present in the system. Because LSb is a decreasing function of oxygen potential, which is reflected in this work by the matte grade, then the mechanism of Sb dissolutions in the slag can be proposed to be, at least partially, oxidic in nature. This conclusion would be valid only for the experimental conditions outlined in this study. It is possible that Sb may dissolve in an oxidic form in addition to the monatomic form suggested by Nagamori.
Bismuth
Distribution coefficients for Bi in the copper matte-slag system were complicated by a saturation effect. The concentrations of Bi in slags were normally within one order of magnitude and repeatable. Bismuth concentrations in the mattes, however, varied over two orders of magnitude, and the values varied considerably even within one sample. Apparently, the level of Bi (0.02 g) added to the approximate 8-g matte-slag charge saturated the system, and excess Bi formed prills in the matte phase.
The concentration of Bi as the only minor element in slags is shown as points in figure 3, compared with the regression line of the data from the previous work. The solubility of Bi in slags is consistently greater when the Bi is present as the only minor element, as compared with the situation when all five minor elements are present. This behavior was consistent at all matte grades and for all slag additives—CaO, Al2O3, and MgO. Bismuth solubility appears to be a decreasing function of matte grade, and therefore oxygen potential, when slag additives are present. Although the data are very scattered, it is possible to conclude that the presence of other minor elements in the copper matte-slag system decreased Bi solubility in the slag as compared with the solubility when Bi was the only minor element.
That Bi saturated the matte-slag system and precipitated as another phase in the matte has been substantiated at the industrial scale. Observations on the behavior of Bi in a typical reverberatory furnace revealed that as the Bi concentration of the matte increased from 180 ppm to > 400 ppm, there was no corresponding increase in the slag Bi concentrations. From that work, it was concluded that the distribution coefficient for Bi was not a constant but increased with increasing Bi concentration. In this case, the distribution coefficient was defined in an expression identical to equation 1. The level of Bi in the system had possibly exceeded that saturation
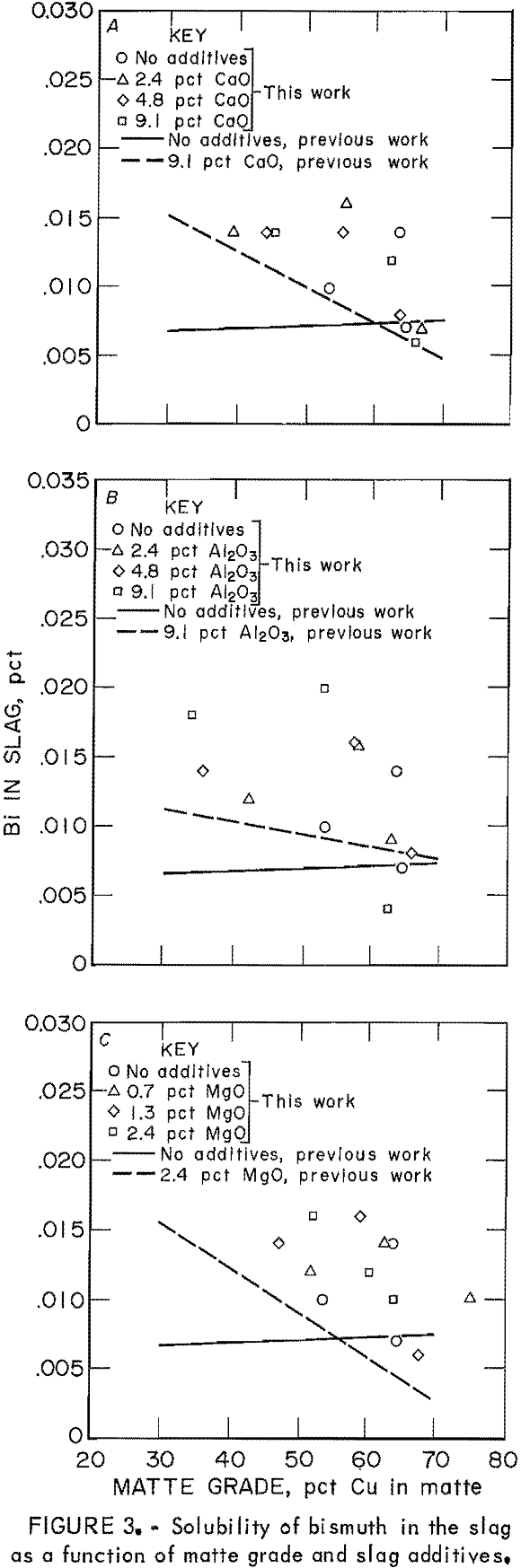
level, and the excess Bi was reporting as another phase to the matte.
Selenium
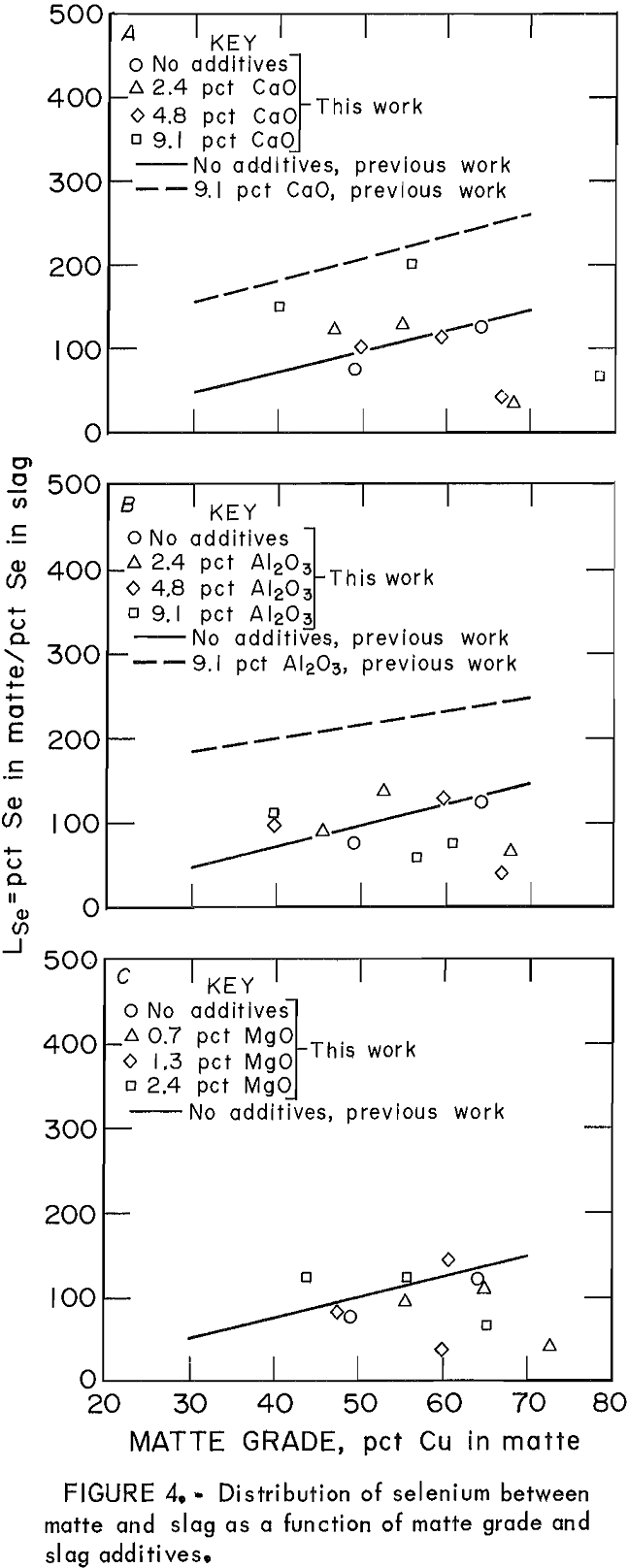
The behavior of Se in the copper matte-slag system can be represented by the distribution coefficient. The data for LSe as the only minor element are shown by the points in figure 4. These can be compared with the data obtained when all five minor elements were present simultaneously, represented by the regression lines from the previous work.. Levels of Se in slags ranged from 0.005 to 0.025 wt pct, while concentrations in mattes were much higher, from 1 to 1.3 wt pct. Thus, distribution coefficient values were large and scattered. In general, the value of LSe when Se was the only minor element was either the same or less than LSe when other elements were also in the system. In the previous work, the effect of high (approximately 9 wt pct) levels of CaO and Al2O3 caused the LSe to increase from 100 to near 200 (at 50 pct Cu matte). When Se was the only minor element, however, the effect of slag additives Al2O3, CaO, and MgO was not significant.
Nagamori suggested that Se has a composite dissolution mechanism in which it dissolves in both monatomic and molecular form. The presence of significant amounts of other minor elements in the matte-slag system increased the LSe values over those obtained when Se was the only minor element present in the matte-slag sample, which indicates that relatively more of the Se present reported to the matte. The presence of other elements in the slag decreased the dissolution of Se from the concentration it would attain were it the only minor element. In this manner, the behavior of Se was similar to that of Bi.
Tellurium
Distribution coefficients for Te throughout this work also were complicated by a saturation effect, in a manner very similar to that for Bi. Figure 5 shows the solubility of Te in slag as
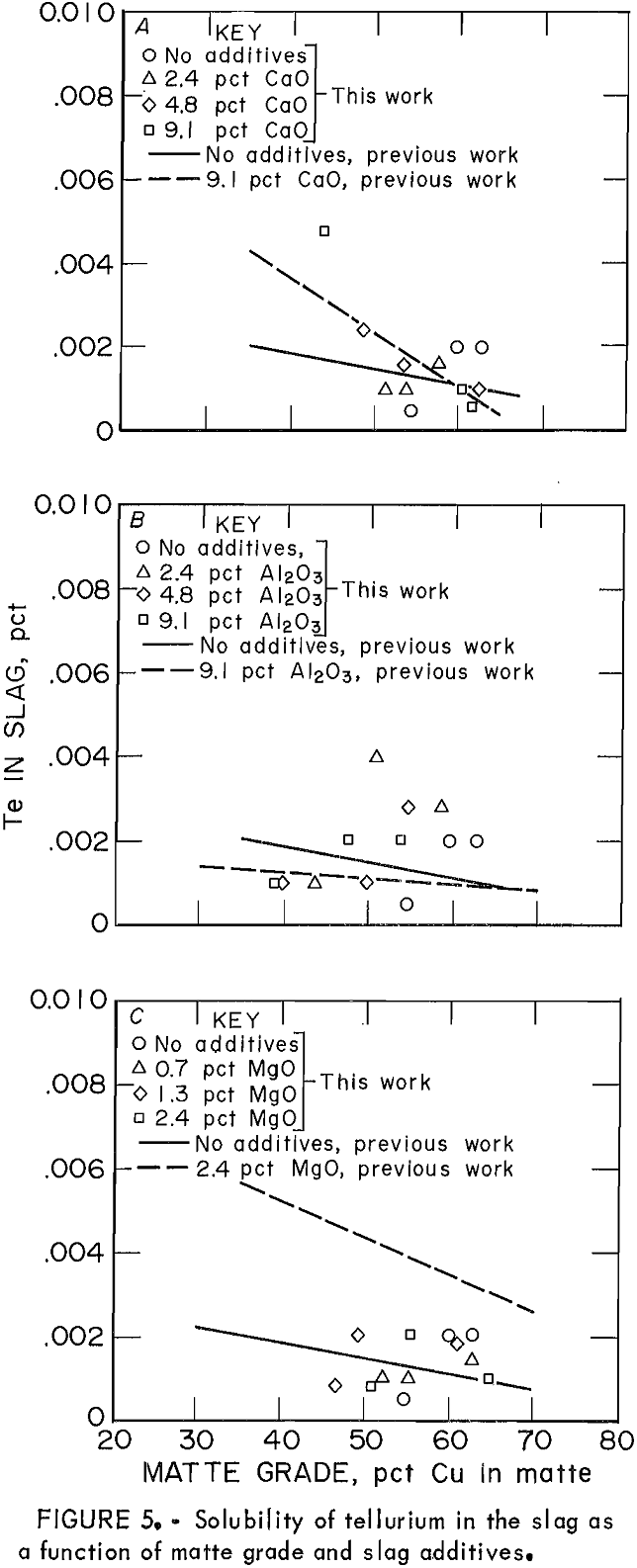
the only minor element in the system (points), compared with its solubility when the other elements (As, Sb, Bi, and Se) are present (lines). In general, the solubility of Te in the slag was not significantly affected by the presence of other minor elements. The effect of slag additives Al2O3, CaO, and MgO on Te behavior was almost negligible.
Mechanisms for Te dissolution In slag are the same as those for Se, both monatomic and molecular. Molecular dissolution is characterized by an increasing solubility of the element with decreasing oxygen activity. This is exactly the type of relationship between Te solubility and matte grade that was observed previously.
Summary
A better understanding of minor-element behavior in copper smelting systems could lead to improved recovery of these elements, more efficient extraction of copper, a more healthful and safer work-place, and less hazardous disposal situations. This work represents further studies by the Bureau of Mines on the characterization of minor element behavior.
The distribution coefficient for As was observed to be independent of matte grade and the presence of the other minor elements. The effect of slag additives on the behavior of As will be discussed in a forthcoming publication. The distribution coefficient for Sb varied only with matte grade and was not significantly altered by either slag additives or the other minor elements. When Bi was present alone, Bi concentrations in slag were consistently higher than when Bi was present with the other minor elements. Slag additives increased Bi concentrations in slags, while increasing matte grade decreased Bi concentrations. The distribution coefficient for Se increased with the presence of other minor elements. When no other minor elements were present, relatively more Se reported to the slag than when the other elements were present also. The behavior of Te, expressed as the solubility in slag, was not significantly different from the single to the multiple element study. Although data were scattered, slag additives appeared to have more effect on Te solubility in the presence of other minor elements.
No discernible interelement effect was observed for As, Sb, and Te, whereas for Bi and Se the presence of other minor elements decreased the relative amount of the single element that dissolved in the slag. This behavior is characteristic of a competition for available sites within the slag. To determine if more specific element interaction phenomena exist, another study must be performed in which only two of the minor elements are present in the copper matte-slag system.
