Table of Contents
The advance made in recent times in this branch of metallurgy is indicated by the attention the subject is receiving from important American Copper producing companies. Reference to the files of publications devoted to the mining industry discloses that some 20 American companies are actively investigating the amenability of their ore, or other material, to leaching methods, and that plants of varying capacities up to 10,000 tons per day are under construction or are projected. Several leaching works are in commercial operation.
It has been frequently pointed out that no method of leaching is universally applicable, for the reason that each ore differs in some particular from apparently similar ore found elsewhere, and also because local conditions are rarely the same even where the ore closely approximates in character that being worked at some other point. For this reason the older companies are approaching the matter in a conservative manner, experimentally determining for themselves the salient features in each instance.
In considering a leaching proposition three factors at once fix the attention:
- disengaging the metal from mineralized forms in which it is found in nature;
- recovering the metal in a commercial state after it has been dissolved;
- the apparatus best adapted to carrying out the several operations.
Bringing Copper Metal into Solution
This feature has been found to present no serious difficulties. Numerous solvents for copper are known, and it has been repeatedly demonstrated on a commercial scale that from 80 to 90 per cent, of the metal contained in an ore can be rapidly brought into solution. It is interesting to note, however, that all of the companies above referred to have selected sulphuric acid as the basis for a lixiviant. Where oxidized ore practically free from precious metals is treated simple leaching with sulphuric acid has been adopted in every case reported, less than an hour sufficing for the operation under favorable conditions.
When the copper to be extracted is found mineralized as a sulphide the use of sulphuric acid as lixiviant necessitates breaking up the sulphur combinations by roasting in order that the metal can be brought into soluble form. This operation, however, instead of being a drawback, presents advantages. The expense of roasting is light, for it is very effectively and cheaply carried out in modern mechanical furnaces, and the lixiviant itself is thereby obtained with which the copper is subsequently removed from associated gangue. In some cases it has been found that the addition of a little salt at certain stages in the roasting assists in bringing copper into solution and facilitates the extraction of any silver present.
The degree of comminution necessary in order to obtain a high extraction depends upon the mineralogical and physical characteristics of the ore. In the case of a sulphide requiring a preliminary roast, reduction to 16 mesh has usually been found sufficient. Where an oxidized ore is concerned, as the copper minerals for the most part lie along fracture planes in the matrix, comminution to ¼ in., or even ½ in., often exposes the cupriferous portion sufficiently to permit satisfactory extraction.
If time is an element of importance, as in the case of small mines where it is not desirable to have considerable capital tied up in ore undergoing treatment, or where a high percentage of extraction is essential, fine grinding accompanied by agitation may sometimes be advantageous; but the benefits accruing from fine comminution disappear where large deposits of low-grade ore are handled. Naturally, the finer the grain, the more readily the acid solution attacks copper minerals, and the less time is required to bring the metal into solution. Then the question arises whether or not it is more economical to crush to ¼ in. and leach in large vats by percolation, or whether the ore should be reduced to 16 mesh, or finer, and subjected to agitation.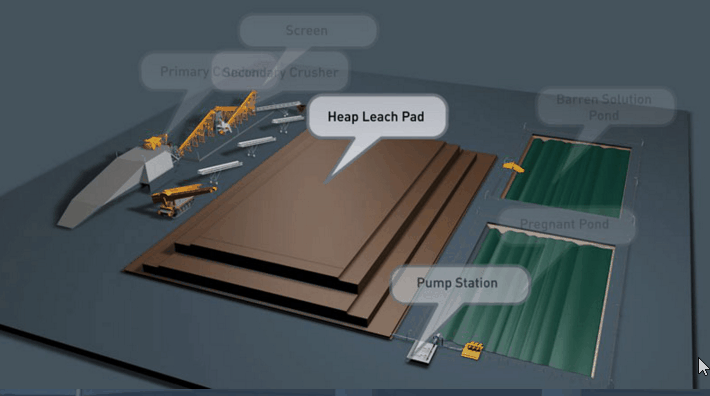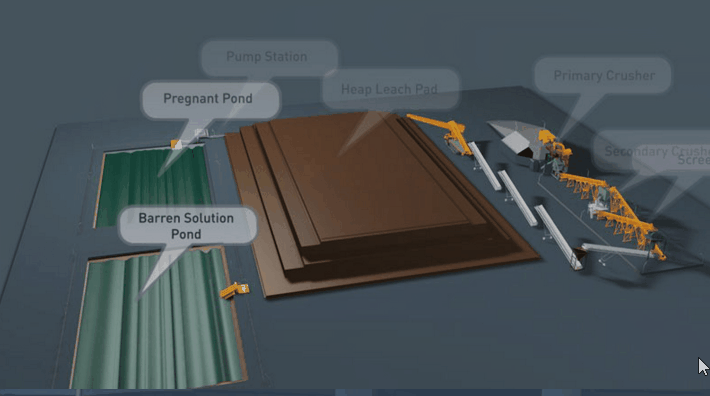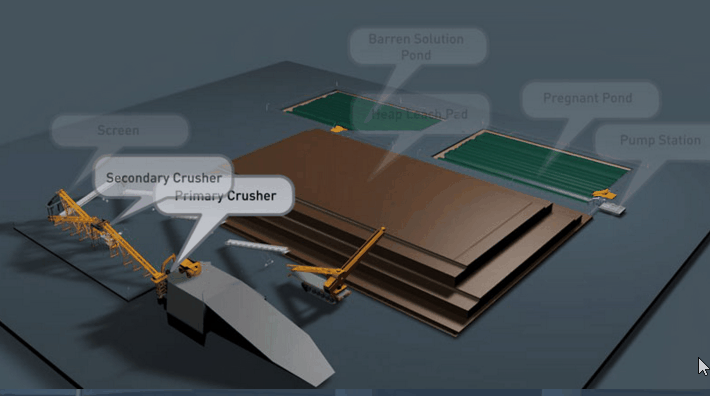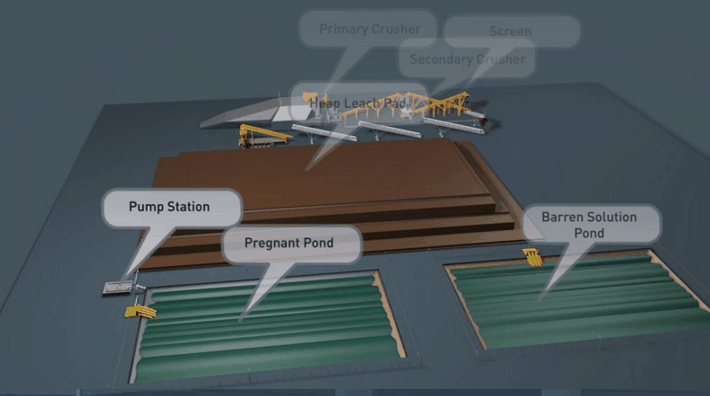
For instance, a certain ore when crushed to ¼ in. was found to yield 70 per cent, of its contained copper in three days by the use of weak acid solution. The same ore crushed to 16 mesh permitted 85 per cent, extraction in 4 hr., using a much stronger acid lixiviant. With a 2 per cent, ore this means a saving of 6 lb. copper per ton of ore treated, together with economy in time. Offsetting these advantages are: greater first cost of crushing and agitating machinery; higher maintenance charges on plant; and difficulty in washing the finer tailings. Almost any ore may be treated by percolation when properly prepared. It has been frequently noted by metallurgical writers that solutions will percolate more freely through a roasted ore than through one treated in its natural state.
Agitation of course brings copper more quickly into solution than percolation, and one advantage of this rapid action is that sulphuric acid solutions manifest a selective action, attacking oxidized copper minerals before the iron and alumina content of an ore. Hence, in leaching by agitation the resultant lixivium is apt to be less contaminated by these elements than where percolation is applied. This is of importance when copper is to be subsequently removed from leach liquors by electrolysis, using cells based upon copper-refinery practice. It is not so important when depolarizers and moving electrodes are employed.
Many oxidized copper ore bodies occur in what are known as contact-metamorphic deposits, the gangue of which is largely composed of garnet and associated minerals, wollastonite, vesuvianite, epidote, etc. Analyses of such ore disclose large amounts of calcium oxide, which may lead to erroneous assumptions and possible rejection of leaching processes as unsuitable in such cases. The facts are, however, that these calcareous minerals are not attacked by weak acid lixiviants in the length of time necessary to extract the associated copper. It is not always safe to base a verdict upon the evidence of analyses alone when considering the appropriateness of this or that method of reduction.
When leaching with sulphuric acid lixiviants, and if the ore contains no sulphides or sulphates, the acid cost may constitute a preponderating proportion of the expense, and a source of cheap acid becomes a vital factor. On low-grade ore treated in the raw state the consumption of acid varies between 2 and 4 lb. per pound of copper produced, and when this reagent costs $0.014 per pound the outlay for this item alone may equal one-third of the total cost of the operation. This expense may be greatly reduced, or even wholly removed, when sulphides are available, for by roasting the latter and passing the gases containing sulphur dioxide into electrolyzing cells the amount of current required to deposit copper is reduced and an excess of acid accumulates for use in leaching new batches of ore. With the exercise of some ingenuity sulphur dioxide may be applied in this manner without delaying the operation and without causing annoyance to those working around electrolytic cells.
In leaching roasted mill tailings the consumption of acid appears to be small—about 50 lb. acid per ton of tailings handled—even with very fine material. This illustrates the benefit derived from roasting substances containing colloids, such as mill slimes, when the copper is to be subsequently extracted by lixiviation. Roasted porphyritic ore also requires very little acid, partly because aluminous colloid compounds are dehydrated in the process, and partly because such ore contains few ingredients soluble in the strength of solutions employed.
Referring again to the fouling of liquors made use of in cyclic leaching, experimental data recently published, as well as unpublished, indicate that the matter is not as serious as was formerly thought. In one instance where considerable quantities of ore containing large amounts of lime and soluble iron were treated experimentally with copper sulphate solutions, ferrous sulphate was present in such quantities that it crystallized in the pumps and pipe lines, at times completely stopping the flow, and yet the results were considered satisfactory; that is, in a series of six tests 1,082 lb. of copper are said to have been deposited, under conditions mentioned, with an expenditure of 1.5 kw-hr. per pound of copper. All of these tests except one were made without use of a diaphragm. The experiments were carried out at the Greenawalt ore-testing plant in Denver.
The height of ore column permissible in percolation has been the subject of much investigation. Garnetiferous ore crushed to ¼ in. percolates freely, even when the coarser material is mixed with much fine. An ore which contains such a proportion of fines that solutions will not pass through it when crushed to ¼ in. and thrown into a vat, can be rendered permeable by dampening and thoroughly mixing before charging. By this operation the finer particles are made to adhere to the coarser, and do not collect in impervious layers, which is apt to occur when the material is charged in a dry condition.
It has been found in leaching an ore carrying large amounts of lime and iron that when the lixiviant becomes weak in acid, basic iron salts and gypsum separate and clog the interstices between the ore grains, causing the flow to cease. This does not take place when sufficient acid is present to retain iron salts in solution. With proper precautions an ore column of average oxidized copper ore 10 ft. or more in height can be percolated without difficulty; in one recently described ore-leaching plant the percolation vats are being built 16 ft. high.
Heating the lixiviant, of course, always renders it more active, but this is not always desirable in copper-ore leaching using sulphuric acid, because under such conditions combinations of elements other than those of copper are attacked and their bases are brought into solution. It is seldom found necessary to heat the liquors in leaching proper, the strength of acid lixiviant used being sufficient to effect solution of the copper at ordinary temperatures.
Most ores amenable to the leaching process carry such small quantities of the precious metals that they can be neglected. Where gold and silver are present in amounts sufficient to warrant their extraction, they are either converted into chlorides in the roasting furnace and afterward dissolved in brine, or other solvents, from which solutions they may be removed by any one of several well-known methods, or else they may be recovered from the residues in separate operations after the copper has been taken out by acid lixiviation.
Recovering Copper Metal from Leach Liquors
There have been many processes brought out for removing copper from solutions containing that metal, and some of these may find application in special cases, but there are only two which have met with extended commercial use—precipitation by means of metallic iron, and deposition by the electric current. The first mentioned still has its advocates, but electrolysis is meeting with more and more favor as that method becomes better understood by men in the field, although the apparatus now in use is still poorly adapted to the purpose.
At the present time the practice so long in use at electrolytic copper refineries is being closely followed, but it is doubtful whether these methods of procedure are best suited to the requirements of ore leaching. The necessity for improvement becomes obvious when the conditions in an electrolytic cell employed in removing copper from an ore lixivium are closely studied. In copper refining the bath is kept fairly constant with regard to copper content and free acid present, fresh metal being taken up from the anodes as fast as that in the electrolyte is deposited upon the cathodes. In ore leaching, on the other hand, copper is being constantly removed from the electrolyte, and the latter has to be returned to the ore for a fresh supply. It is manifest, therefore, that conditions differ essentially in the two operations and the premises seem to call for modifications of the apparatus used.
In treating an ore lixivium, at the electrode where current enters the bath an acid radical (SO4) is being constantly disengaged which decomposes water and combines with the hydrogen set free to produce H2SO4. This reaction is made apparent by the formation of oxygen bubbles on the anode; if these are not in evidence it is because the gas is entering into combination with some salt or element present in the electrolyte, a feature usually, though not always, detrimental to the process. Nascent oxygen produced in this way may attack the material of which the anodes are composed, wasting them, or it may oxidize ferrous salts in the electrolyte to ferric, thereby bringing into action a solvent which corrodes the copper being deposited on the cathodes. Ferric salts are then being formed at the anodes and reduced to ferrous at the cathodes, wastefully consuming electric current. Or anions and cathions liberated at their respective poles may set up counter currents working in opposition to the main current. To overcome complications introduced by formation of ferric salts attempts have been made to keep the anolyte separated from the catholyte by means of diaphragms, but this recourse has not met with much success in commercial operations.
It is therefore evident that in electrolysis of copper lixiviums there is urgent need of a cell in which oxygen forming at the anodes may either be removed as fast as produced, or rendered innocuous by chemical combination, operations to which the type of electrolytic bath found to meet the requirements of copper refiners does not readily lend itself. Ingenious forms of apparatus have been devised by means of which the liberated gases are shaken from the electrodes as fast as set free, and corrosion of lead anodes is said to be thereby reduced to about 10c. per ton of copper deposited. This is an advance in the right direction, but in such cells impoverishment of the electrolyte at the cathode still assumes adverse proportions, and solutions have to be returned to the leaching department with copper content only slightly reduced.
In electrolysis, economy is affected by facilitating the supply of cathions to the negative electrode so as to avoid as far as possible waste of current in doing work other than that desired. If left to the magnetic attraction of the electrodes alone the movement is too slow to prevent impoverishment and its attendant evils, at least in commercial electrolysis of ore lixiviums.
Circulation of the electrolyte is the means usually taken for supplying necessary copper ions to the cathodes; the more rapid the movement, the more efficient the supply. Also, adhesion of oxygen bubbles to the anodes is lessened by a current of electrolyte impinging against them. Circulation alone, however, does not go far enough, as is apparent from the fact that only comparatively rich copper lixiviums can be treated in the common type of electric refining cells, and that the liquors must be returned to the ore-leaching department in some instances when only about one-fifth of the copper content has been removed. This method of operation calls for continuous pumping of liquors, which serves no useful purpose other than taking up a small amount of copper in one department and depositing it in another, both operations being incomplete. More of the copper than was just stated may under favorable circumstances be removed from a rich lixivium in a refiner’s cell, but only by applying a low current density, which means a large plant for a given production. For example, an electrolyte may be reduced from 5 to 1½ per cent, or less, but the first cost of such a plant will be large. The desideratum is a cell which will rapidly deplete a 1½ per cent, electrolyte to about 0.3 to 0.4 per cent, with the same energy efficiency as in the former case, turning out at the same time a high-grade metal.
Experimental data on a fairly large scale have been obtained through application of the two factors—moving electrodes and depolarizing agents—which give a hopeful aspect to the solution of the problem involved. It is practical to get rapid deposition of metal of excellent quality from impure lixivium, but at the expense of power. However, considered from the commercial standpoint, results ranging from 1.2 to 2.3 kw-hr. per pound of copper deposited are encouraging. Energy efficiency must, of course, always be taken, into account in addition to current efficiency when the respective merits of different cell types are considered, but first cost and speed are also commercial factors; the greater the current density, the less the size of electrolytic plant.
Deposition of metal on cathodes is affected by current density perhaps as much as by any other factor. This is the same as saying that deposition is in a large measure dependent upon the amount of copper carried by the electrolyte, or which can be brought into contact with the cathodes in a given unit of time, for current density must be progressively reduced as the electrolyte is depleted of its copper if pulverulent precipitation is to be avoided. A firm deposit of copper may be obtained at current densities running into thousands of amperes per square foot, provided impoverishment of the liquors immediately adjacent to the cathodes is prevented. Arborescent accretions also are markedly absent when circulation is properly adjusted to current density.
There are other well-known causes which bring about disturbances during electrolysis, but if copper ions are supplied to the cathode as fast as needed, thereby focusing the current on the work desired of it, irregularities disappear. Whatever the origin of pulverulent deposition may be thought to be, the fact remains, that a firm bright deposit of copper can be obtained from an impure (1.5 per cent.) electrolyte when copper ions are properly supplied to the cathode, and provision is made for counteracting the injurious effects produced by liberated gases, whereas the same liquor in the electrolytic refining type of cell gives only a loose, dark-colored spongy mass which bridges the electrodes and causes short circuiting.
Heating the electrolyte is a questionable procedure, because it renders the objectionable components of the liquor more active in corroding the deposited copper. It has long been known that a greater quantity of copper can be deposited by the same amount of current from a neutral copper sulphate solution than from one of the same metallic content but carrying in addition free acid. The cause of this is that the acid redissolves the deposited copper. If ferric salts are present corrosion is still more marked. These reactions are furthered by elevating the temperature of the bath. Heating an electrolyte always facilitates passage of the current; but ore lixivium contains a variety of salts, and as all ions may serve the current as a means of transport, a large amount of electricity may find passage without depositing copper. A good conducting electrolyte is not necessarily economical in deposition of metal.
Copper Leaching Equipment
Concrete as a material for construction of vats, launders, etc., in leaching works has found application a number of times, but nothing is on record as to its durability under working conditions. As the cement binder is alkaline in its nature, and as leaching solutions are of varying acidity, it is to be expected that where the two come into contact chemical reactions must necessarily follow. In a measure the strength of the acid liquors will have an influence, as will also the salts contained in the solutions, but where much free acid is present ordinary concrete will crumble.
Concrete has manifest advantages over wooden construction, especially in hot, dry countries, and, of course, concrete vats are less apt to deteriorate than are wooden ones in case of temporary suspension of activities. To make the good qualities of concrete available the problem is to devise means for preventing penetration of acid liquors into the body of the concrete, which sooner or later must result in its destruction.
There are several ways in which this object may be accomplished on a small scale, but their adaptability to commercial purposes has yet to be demonstrated. The inner layer of the concrete may be made of some acid-resisting composition, or a concrete vat may be lined with brick which are not affected by the solutions, or after the vat is completed a non-corroding coating may be applied which sinks into the finished concrete and protects the binder. Heavy mineral oils, and paraffin applied hot, have been used in this manner. Coatings of pitch or asphaltum do not seem to answer the purpose well, for sulphuric acid solutions penetrate them through minute holes, forming gypsum, with consequent swelling, and the asphalt coating peels off as the concrete disintegrates.
Recently it has been stated that reinforced-concrete vats may be lined with mastic asphalt and satisfactory results obtained. The asphalt mixture had to be specially prepared and the work was done by workmen skilled in handling such material. It remains to be seen how this construction will endure under working conditions on an extended scale. It would seem to be subject to the defect common to all linings designed to protect corrodible material from the action of strong acids: if a leak starts anywhere the alkaline concrete binder will be attacked as a matter of course and this will only become known when the structure collapses.
Mixing heavy mineral oils with the concrete as it is made, affords a hard liquid-repelling mass which behaves well with weak acid solutions. The oil is incorporated with the cement-sand mortar before the broken rock is added, as in the L. W. Page waterproofing process. This method renders the whole mass to some extent acid-resistant in that the oil repels liquors seeking to permeate the structure. It has the advantage over the lining system that leaks are self-stopping and the lining is not pushed off leaving the concrete backing unprotected. Still, even oil concrete slowly disintegrates when strong (10 per cent.) acid liquors are permitted to act on it for weeks at a time.
Certain kinds of wood—notably the Western fir—have been found to resist acid solutions to a remarkable degree, even without lining. Storage vats holding cupriferous solutions containing 10 per cent, or more free sulphuric acid showed no signs of failure after being in use for several months.
With the help of reinforced concrete, leaching vats of large dimensions may be constructed. In a plant now being built the vats are designed to hold each 10,000 tons of ore. It is expected that two days’ percolation will suffice to render soluble 90 per cent, of the copper contained in this ore.
Copper Leaching Costs
Extraction of copper from suitable ore by leaching methods is generally assumed to cost less than the combined expense of concentration, smelting, and refining, and experimental data tend to confirm this opinion. At the Butte-Duluth plant, which is handling in excess of 100 tons of oxidized ore daily, the copper is said to cost $0.085 per pound. This figure probably refers simply to working expenses and does not include freight East, marketing, etc. After the contemplated increase in the capacity of the plant it may be expected that cost of the metal will be materially reduced.
Experimental work carried out at Morenci, Ariz., indicated the probable cost of leaching tailings slime at $0.0743 per pound copper delivered in New York. This includes expenses after the copper leaves the mine, with due allowance for interest and depreciation.
From extended tests made at the Anaconda works, carried out in great detail upon a working scale, the conclusion was reached that copper could be produced from concentrating-mill tailings for about $0.07 per pound copper.
At the Steptoe mill, in Nevada, a small leaching annex is treating oxidized copper ore in conjunction with flue dust at a cost of $0.08 per pound copper recovered.
The actual cost of copper produced by leaching methods is, however, not of so much importance as the fact that ore may be handled in this manner which is not amenable to reduction by any other process. A siliceous oxidized copper ore carrying from 1.5 to 2.0 per cent, metal may be profitably leached under favorable conditions; there are no other metallurgical means known for commercially handling ore of that character and grade.

Copper Leaching Practices
For centuries small amounts of copper have been recovered from acid mine-drainage waters. In recent years, the expansion of copper-bearing waste dump leaching for copper recovery has established a technological basis that is rapidly converting the art of copper leaching into a science.
Present method of establishing leach dumps by truck haulage does not permit uniform wetting of the material for proper leaching. Material spilled over the dump crest segregates as it roils down the dump slope, and the higher the dump, the greater the degree of segregation. Exceptions to this type of operation are practiced by the Anaconda Company at their Butte, Montana operation and by Ranchers Exploration and Development Company at the Bluebird Mine in Miami, Arizona.
Current technology in mine dump preparation being practiced successfully by various operations in the leaching of copper is as follows:
- Removal of all vegetation in area to be overdumped.
- Compaction of area, and emplacement and compaction of two feet of clay material for a base.
- When economically possible, removal of “fines” from mine waste before emplacement in dump area.
- Limit the widths of the dumps of sulfide – containing material where possible to 400 feet, thereby permitting the maximum size of width that will still provide natural aeration for oxidation of the sulfides.
- Removal of the top two feet of material from the dump surface which is “fines” generated by the wheel action of the haulage trucks.
- After removal of the surface “fines”, rip the dump surface to a depth of eight to ten feet.
- Moisten waste material as it is being placed on the dump to start the oxidation of the sulfide minerals while ample atmospheric oxygen is available.
- If economically feasible, construct dumps to heights of 50 feet and leach before emplacing a subsequent “lift” on the dump. Field experience with various operations indicates substantially the same copper content of solution is obtained with 50-foot high copper sulfide containing dumps, and 20-foot high oxide containing dumps as with higher dumps.
- Mine material once dumped in an area to form a dump or heap will result in that dump or heap having a 40 percent void space. After a single year with or without leaching, this material compacts to approximately 30 percent void space.
Solution distribution in dump leaching follows a variety of approaches These approaches involve sprays, ponds, injection holes, and irrigation ditches. Some companies control the pH of their leach solutions while others merely recycle the solution on an “as is” basis from their cementation process.
Several techniques are used in the distribution of leach solutions onto waste dumps or heaps. These techniques involve:
- The construction of pond areas which are subsequently flooded.
- Construction and operation of spray systems.
Currently, Bagdad Copper and Rancher’s Bluebird mine are both using the General Mills solvent extraction process to recover copper from solution. All other operations are using the cementation process of precipitating copper on salvaged “tin cans.”

