Copper hydrometallurgy the leaching of siliceous low-grade copper-bearing ores and waste rock that cannot be processed economically by milling and concentrating is an important low-cost process for recovering copper. In the free world, the United States is the principal user of this process, and in 1965 nearly 10 percent of the U.S. production of primary copper came from dump and in-situ leaching of sub-mill-grade material. In the free world, including the United States, such leaching accounted for 150,000 tons annually, or 3 percent of the 5-million-ton total for primary copper produced in the mid- 1960’s. The production of primary copper by leaching is expected to increase five fold by 1975.
In the United States, the production of primary copper by dump and in-situ leaching of sub-mill-grade material increased 359 percent from 28, 531 short tons in 1946 to 131,000 tons in 1965. All other primary-copper production employing some heap and vat leaching, but principally milling and concentrating of ores, increased 122 percent from 608,737 short tons in 1946 to 1,351,734 tons in 1965. This report reviews the methods currently in use or under study in the United States for the recovery of copper by hydrometallurgy.
Chemistry of Leaching
Ferrous sulfate and sulfuric acid slowly form when water containing dissolved air contacts broken rock containing pyrite. In the presence of additional oxygen and sulfuric acid, ferrous sulfate is slowly oxidized to ferric sulfate. Several strains of oxidizing bacteria not only thrive in high-acid copper solutions but accelerate the oxidation of sulfide minerals to form acid and soluble copper and iron sulfate. Thiobacillus thiooxidans bacteria utilizes the oxidation of the sulfur to form sulfides as their energy source, and Thiobacillus ferrooxidans oxidizes ferrous iron to ferric iron. These bacterial strains complement each other in that the former produces acid which keeps the ferric iron produced by the latter in solution. This forms acidified ferric sulfate, a powerful lixiviant for copper minerals.
Leach solutions containing acid only can dissolve most of the oxide-copper minerals. When such minerals are leached in the absence of pyrite, sulfuric acid must be added to the leach solution in amounts ranging up to five times the weight of copper dissolved.
In the presence of dilute sulfuric acid and ferric sulfate, all copper minerals are dissolved although some minerals are dissolved slowly, often at a rate of only 1 percent per month.
Recent studies by the Bureau of Mines on chalcopyrite, the principal copper mineral in most porphyry-copper mine waste dumps, show that the dissolution rate increases twenty fold if chlorine or sodium hypochlorite is added to conventional sulfuric acid leaching solutions.
Factors for Successful Leaching
The factors necessary for successful leaching follow:
- The materials to be leached should have a low content of acid-consuming gangue and basic minerals.
- The copper-bearing minerals must be in thorough contact with the leach solution.
- The copper in the solution filling the interstitial space must be maintained at a low concentration to speed the diffusion of the high-copper- content solution from the voids of the rock particles.
- The surfaces of the particles should be sequentially wetted and dried to increase contact with oxygen and to bring the pregnant solution from the voids of the rock particles by capillary action.
- The acid concentration in the leach solution should be adjusted to attain a minimum dissolution of iron while obtaining a maximum copper loading.
Underground Leaching
Conventional
Underground in-situ leaching is used in areas surrounded by tight and impermeable rock to dissolve copper from fractured rock and the gob remaining after the termination of mining by block-caving or other methods.
Worked-out scopes in deep mines are either flooded with barren leach solution or are permitted to fill with indigenous acidic mine waters. Sub-sided capping remaining above worked-out block-caving sites is supplied with leaching solution distributed by gravity from ponds and drill holes.
The pregnant solutions produced by underground leaching during a 5- to 6-month-contact period are usually collected in abandoned adits and pumped to storage ponds on the surface adjacent to the copper-precipitation equipment. The retention of pregnant solutions in these ponds partially removes slimes and clay originating in the gob left after mining and from the mill tailings used for the backfilling of stopes.
The Anaconda Co. uses a flocculent and either inclined or vertical thickeners to remove solids and colloidal matter from waters pumped from the Mountain Con and Steward underground mines before applying the clarified mine waters plus added acid to the Berkeley leaching dumps.
Project Sloop
In October 1967, the Kennecott Copper Corp. disclosed details of a proposal made to the U.S. Atomic Energy Commission for a joint experiment called Project Sloop in which a low-grade copper ore deposit near Safford, Ariz., would be fractured by a contained underground nuclear explosion prior to in-situ leaching.
The total cost of the three phases of the experiment is estimated at $13,175,000. Phase 3, a 3-year study costing $6,675,000, would use 2,600 gallons of leach solution each minute following underground reentry and rehabilitation activities. In phase 3, industrial radiological safety problems would receive major attention while concurrent investigations would include the evaluation of solvent extraction methods, the electrolysis of solutions of cement copper in sulfuric acid, and conventional smelting and electrorefining methods.
The experiment, if approved, could result in a new mining technology in which the surface is left undisturbed and the expense and large amounts of power involved in the conventional mining of waste material and ore are eliminated.
If Project Sloop proves feasible, the estimated 75 million short tons of copper contained in U.S. ores averaging 0.86 percent copper could be supplemented by another 58 million short tons of copper in minus 0.47 percent material.
Estimated Costs for Nuclear Fracturing and In-Situ Leaching
Preliminary calculations indicate that the direct average total cost for nuclear fracturing alone would be 12.8 cents per ton of ore fractured using a 100-kiloton device, and 55.2 cents per ton using a 10-kiloton device. It may be economical to use nuclear fracturing and recovery by in-situ leaching for deposits containing as little as 4 pounds of recoverable copper per ton of material. Copper production could begin in less than one-half the time required by conventional mining methods.
The estimated capital expenditure for copper precipitating facilities alone ranges from $1,000,000 for a 10-million-pound annual capacity to $200,000 for a 1-million-pound annual capacity.
The estimated cost of copper production during a 10-year leaching operation following nuclear fracturing ranges from 17.0 cents per pound of copper produced by a 10-million-pound annual operation to 24.4 cents by a 1-million-pound annual operation. To these costs must be added the cost of the nuclear fracturing of the deposit, which may range from 0.8 to 13.8 cents per pound of recoverable copper depending on the copper content in each ton of ore and the yield of the nuclear device used.
A report issued in 1959 by the Colorado School of Mines Research Foundation included the following estimated costs related to a hypothetical situation for nuclear fracturing and in-situ leaching of a 450-foot-thick, 50-million-ton ore body:
Atomic device in place……………………………………………………………………..$3,500,000
50,300 feet of leach solution input holes……………………………………………..1,800,000
Rubber-lined pumps and pipes……………………………………………………………..285,000
Installation cost…………………………………………………………………………………..220,000
Development of one shaft and 45,000 feet of Lateral work……………………2,327,000
No estimated costs were shown for the cementation equipment and its ancillary facilities.
The complete evaluation and economics of a nuclear-fracturing and leaching operation await the completion of a full-scale test to determine the percolation rate of solution, the copper-recovery rate, the cost of radiation safety measures, and the time for radioactivity decay to reach a level permitting safe leaching and copper processing.
Dump Leaching
Sub-mill-grade waste rocks overlying some copper ore deposits have been depleted of copper by the action of bacteria, owing to gradual oxidation of the sulfide-copper minerals and leaching. Much of this soluble copper has been carried by water downwards hundreds of feet to enrich sulfide-copper ore deposits.
The sulfide-copper content in the waste ranges from cutoff grade to zero at any particular mine; however, the oxide-copper content of some waste may be greater.
More than 60 percent of the solids produced in U.S. copper mines are discarded either because their copper content is below milling grade or they are not amenable to flotation treatment. Most of this waste is generated at surface mines where in 1966 over 0.37, billion short tons were added to an existing accumulation of 5 to 10 billion short tons.
The leaching of some waste dumps is hindered by the slow dissolution rate of some iron-copper sulfide minerals and by the cementing and coating of the waste surface by iron precipitates and clay.
A process developed at the Kennecott Research Center has won U.S. Patent 3,330,650. The process will be used to purify a portion of the solution from the precipitation plant by removing aluminum and regenerating sulfuric acid. Iron is removed from the other portion of the solution by a change in pH. The two purified solutions are recombined to form the optimum composition for extracting copper from the mine waste.
Pregnant solutions are pumped to storage ponds located near the copper precipitation equipment. In several situations, bentonite or plastic film has been used to line the bottom and sides of storage ponds to prevent seepage.
Copper recovery by dump leaching in any one year is not proportional to the copper content of the leachable sub-mill-grade rock placed on leaching dumps in the same year. This is because time is required for some of the copper and iron minerals to oxidize enough to produce a condition favorable to leaching. Leaching continues for many years after the last of the leachable rock has been mined from an open pit.
In 1964, 125,000 short tons of copper were recovered by dump leaching. This recovery required the processing of about 42,000 U.S. gallons per minute (60 million U.S. gallons per day) of solutions averaging about 1.5 grams copper per liter (12.5 pounds copper per 1,000 U.S. gallons).
In 1965, 14 large copper-leaching operations produced pregnant solutions containing 1 to 12 ppm U3O8 (4 ppm average). Six of the mines, producing half of the total leach solution, had an average grade of 10 ppm U3O8, which is equal to 1,600 pounds of U3O8 per day. With the completion of expanded copper-leaching operations in the next few years, as much as 6,000 pounds of U3O8, a day may be available from these sources.
Cementation Equipment
As recently as 1965 most of the conventional cementation (copper-precipitating) equipment comprised either gravity, rotating drum, or activated launders, most of which had substantial labor requirements. The colloidal-size copper particles precipitated in rotary drum launders added to the operational problems and costs.
The Kennecott Copper Corp. recently eliminated these disadvantages in its operations by the development of a new cementation system. Operating experience with this system demonstrated that the application of kinetic principles results in an easily filtered, 90- to 95-percent-copper product, using less scrap iron for each pound of copper precipitated than in the conventional equipment. This high-capacity system features automatic control and mechanized material handling. By use of the new continuous and self-cleaning system, 99 percent of the copper is precipitated from solutions containing 0.4 to 4.0 grams copper per liter.
The Phelps-Dodge Corp. recently developed a V-trough precipitator for use at Bisbee, Ariz., which uses sponge iron to precipitate the major portion of the copper in solutions produced by dump leaching. Scrap iron precipitates the remaining soluble copper and filters fine-grained cement copper from the partially decopperized solution.
The V-trough precipitator minimizes the former caking and blinding problems encountered when using sponge iron in static launder-type precipitators.
Consumption of Iron and Sulfuric Acid
The consumption of iron and sulfuric acid is a major factor affecting the cost of leaching, followed by precipitation of soluble copper by scrap iron or sponge iron.
A high acid content in pregnant solutions increases the consumption of iron in the precipitation equipment, while a high acid content in the leaching solutions applied on the dumps increases the consumption of acid by gangue or basic minerals.
Generally, during the leaching of sulfide-copper minerals in waste dumps, 16 to 17 pounds of iron equivalent is precipitated on the broken solids from each 1,000 U.S. gallons of leach solution applied. Collectively, this iron equivalent is derived from the iron used for cementation and the iron dissolved by passage of solution through the waste solids. The precipitated iron, a slimy mixture of basic ferric sulfates and ferric hydroxide, often contains clay, elemental sulfur, and bacteria. At some dumps, where the leach solution is supplied from a covering pond, it is necessary to periodically rip and remove the settled slimes on the bottom of the pond to maintain uniform percolation.
Acid
Several large leaching operations use sulfuric acid produced nearby from waste smelter gases, whereas a few smaller leaching operations use acid made from elemental sulfur or pyrite concentrate. Small amounts of suspended iron sulfates and other impurities in the acid used for leaching are not objectionable.
Sponge Iron
Sponge iron is used in one cementation system as the principal precipitant, supplemented by detinned cans as the final precipitant. However, the static beds of sponge iron become cemented and caked, and the surface of the iron quickly becomes coated with copper. This prevents further contact of the iron with the solution. The Phelps-Dodge Corp. eliminated this problem by the development of a V-shaped precipitation vessel so designed that the input solution levitates and abrades the sponge iron particles.
Based on the consumption of iron, the V-trough precipitator using sponge iron is as efficient as conventional precipitation equipment using detinned cans.
Sponge iron is used advantageously by another copper producer for the leach-precipitation-flotation (LPF) processing of an ore containing about equal amounts of oxide- and sulfide-copper. The resultant cement copper and the nonreacted portion of sponge iron comprise a portion of the solids processed in the sulfide-copper flotation recovery circuit.
Sponge iron is usually produced by the consumer, using pyrite concentrate recovered from mill tailings as the feed material that is roasted to produce a calcine. Reduction of the calcine yields a product containing up to 75 percent total iron, including 35 to 50 percent metallic iron. The cost of producing sponge iron in a 100-ton-per-day plant in 1958 was about $13.60 per short ton ($34 per short ton of contained metallic iron).
In 1963, the Phelps-Dodge Corp. developed a process for producing sponge iron from high-iron-content converter slag. The molten slag is granulated in a water bath and, after drying, is reduced by a countercurrent stream of reformed natural gas to yield sponge iron. This product contains 55 to 60 percent metallic iron and up to 5 percent copper that is recoverable in the cementation process.
Kennecott’s Western Mining Division prevents the reoxidation of the hot reduced sponge iron by the presence of incandescent coke during its discharge from the reduction vessel and during the cooling cycle.
Scrap Iron
Scrap iron, having a low carbide content and a large surface-to-weight ratio, is almost universally used for precipitating copper from solutions even though it is expensive and is in short supply in some localities. Scrap cans must be burnt to remove coatings before shredding. One Utah copper producer now uses the product from a nearby plant that shreds 350 tons of steel scrap in 8 hours.
Theoretically, 1.00 pound of iron will replace 1.37 pounds of copper in acid solution. In actual operations, 1.3 to 4.0 pounds of metallic iron are consumed for each pound of copper precipitated from solution because of the formation of ferrous sulfate by the dissolution of scrap iron by both acid and ferric sulfate.
Scrap iron does not reduce the copper content in 1 liter of solution to less than 60 milligrams unless the reactants are agitated.
Cost Data for Conventional Practices
General
The total cost for producing copper by in-situ leaching, cementation, and subsequent conventional smelting and electrorefining is generally 5 to 15 cents a pound lower than for copper produced by the conventional open-pit mining, milling and concentrating, and smelting and electrorefining route.
The cost of labor directly related to the leaching and cementation operation is about one-tenth the value of the copper recovered by this method.
One gallon of cupriferous solution flowing continuously for 1 year and yielding 1.0 gram of copper from each liter of solution 18.3 pounds from each 1,000 U.S. gallons) will supply 4,380 pounds of copper having an annual value of $1,664, based on copper at 38.0 cents per pound. The total direct cost of leaching and precipitating copper from solution is less than 10 cents per pound.
Peruvian Mine Waters
Based on a Peruvian operation in the early 1960’s, the operating cost for cementation only was 3.0 + 1.25/q cents per pound of copper precipitated, where q = copper content in grams per liter of pregnant solution. This low cost is attributed to small expenditures for labor, power, and equipment, and a low ratio of dissolved iron to copper.
In-Situ Leaching in Arizona
At one U.S. operation 9,000 short tons of copper equivalent is currently produced annually by the in-situ leaching of waste rock and ore remaining after the termination of block-caving mining. The copper is precipitated from solution, in the form of cement copper containing 70 to 80 percent copper, by scrap iron.
The approximate costs of inputs in cents per pound of contained copper so recovered follow:

Additional expenditures to cover amortization, freight, maintenance, overhead and general expense, insurance, and taxes increase the total cost of leaching and cementation to about 10 cents per pound of recovered copper.
Cost of Marketable Copper from U.S. Dump Leaching
In several operations of varied capacity the total production cost of refined copper, originating from the dump leaching of waste rock principally containing sulfide-copper minerals or of selected waste rock and sub-mill-grade ore principally containing oxide-copper minerals, ranges from 13 to 22 + 1.25/q cents per pound, where q = copper content of the pregnant solution in grams per liter.
These total production costs include amortization, freight, operation and maintenance expenses, refining and marketing, general company expense, fringe benefits, insurance, taxes, and safety.
The production cost is near the high side of the range for one Arizona operation where oxide-copper minerals mixed with calcite are leached by solutions containing up to 5 pounds of added sulfuric acid, made from Gulf States elemental sulfur, for each pound of copper recovered. The consumption of shredded cans for precipitation in such an operation is often as much as 3 to 4 pounds per pound of copper recovered because of the greater dissolution of iron-bearing minerals during dump leaching.
Solvent Extraction of Leach Solutions
Ideology
In recent years, much attention has been given to the production (in marketable condition) of the copper extracted during the leaching of waste copper minerals without recourse to cementation followed by conventional smelting and refining.
The ideal process would include solvent extraction of the copper from low-content pregnant solutions followed by stripping of the copper from the solvent by a sulfate-sulfuric acid solution. This would produce an acceptable low-iron-content feed solution for electrolytic cells, preferably containing a minimum of 10 to 25 grams copper per liter of solution.
The solvent should be selective for copper but not for iron at the natural low pH of the pregnant solution. The solvent should have low water solubility and should be nontoxic to those bacteria beneficial to the leaching process. The solvent should be stable at 0° to 80° C but not detrimental to the electrolytic process. The active reagent in the solvent mixture should be compatible with a cheap diluent, such as kerosene.
The solvent extraction of pregnant solutions containing insoluble material is not feasible even though low pH significantly decreases emulsion formation.
A low solvent loss is important in making any product selling for less than $1 per pound since only a few cents per pound of metal recovered can be allowed for lost solvent. Where aqueous solutions are very dilute, this cost rules out solvent extraction entirely because even a trace loss of solvent is unfavorable.
The problem of organic losses is further clouded by the lack of a simple analytical method to determine how much of the costly reagent is lost by entrainment and solution in the raffinate. Other questions bearing on the subject involve the change of copper-loading capacity, the degradation of the active compounds during long use, and the destruction of bacteria, beneficial in the oxidation of copper-sulfide minerals in the waste dumps, by organics contained in the recycled raffinate.
Carboxylic Acids and Naphthenic Acid
Carboxylic acids and naphthenic acid are cheap solvents for extracting copper from low-iron-content solutions with pH above 4.0 and 6.0, respectively. A modified carboxylic acid, α bromo lauric acid, can extract copper at 3.3 pH because of its more acidic character.
All carboxylic acids have the disadvantage that the hydrogen ions produced in amount stoichiometrically equivalent to the metal recovered during extraction must be neutralized. Even when using limestone, as it seems possible to do with a bromo lauric acid, the cost of alkali would be unacceptable when recovering copper from leach solutions containing free acid. In one investigation using naphthenic acid as a solvent, the cost of the naphthenic acid was 0.14 cent while the cost of the alkali (as caustic soda) was 5.6 cents per pound of copper recovered.
LIX-63 Reagent
A few years ago, General Mills, Inc., developed LIX-63 reagent, an α hydroxime specifically useful for extracting copper from solutions produced by the conventional ammoniacal leaching of oxide-copper ores (in closed vessels). This reagent has a low solubility in water and a high specificity for copper but, because it cannot extract copper below 3.0 pH, it is not applicable for extracting solutions resulting from waste dump leaching.
LIX-64 Reagent
More recently, General Mills, Inc., developed another reagent, a mixture of 2-hydroxbenzophenoximes, known as LIX-64. The $2.50-per-pound reagent diluted with nine volumes of kerosene, in countercurrent, four-stage contact with a typical dump leach liquor, attained a loading of 2.42 grams copper per liter of solvent mixture. The raffinate contained 0.03 gram copper per liter. Copper extraction exceeded 96 percent despite the decreased extraction efficiency caused by a decrease of pH during extraction.
The extractive power of this solvent mixture ranges from very strong for Cu+² slight for Fe+³, very slight for Mo+6 and V+4, to nil for other metallic
In early 1967, pilot plants using 6 to 7 volume-percent LIX-64 in kerosine as solvent were operating near the Bagdad, Esperanza, and Inspiration mines. Based on 1,000 U.S. gallons of pregnant leach solution processed, the organic-solvent loss at Bagdad in a system operating at 50° C was 0.139 gallon and was valued at 1.7 cents per pound of copper recovered; at Esperanza the Loss was 0.10 gallon.
Bagdad Copper, in a joint venture with the Chemetals Corp., plans to make copper powder by hydrogenating the copper-acid solution produced by stripping the loaded solvent. In March 1967, Esperanza made its first 40-ton shipment of cathode copper from a four-cell electrolysis installation in which only 10 percent of the copper was plated out of electrolyte containing 50 grams copper per liter.
Cost Estimates Using LIX-64 Reagent
Recently published cost estimates were based on laboratory and pilot plant studies using a solvent mixture of LIX-64 reagent diluted with nine volumes of kerosene to extract leach solutions containing 1.0 to 2.0 grams of copper per liter (8.3 to 16.7 pounds per 1,000 U.S. gallons). The loaded solvent was then stripped to produce solutions suitable for electrowinning.
One estimate shows the cost of solvent extraction facilities alone as $420,000 for a plant capable of recovering 10 tons of copper per day from about 2 million gallons of solution. Depredation charges would range from 0.56 to 0.70 cent per pound of copper recovered. A full-sized extraction plant for handling 3,300 gallons per minute of leach solution at Bagdad was estimated at $1,980,000.
Estimated operational costs, in cents per pound of copper contained in the feed electrolyte supplied to the electrowinning section, follow:
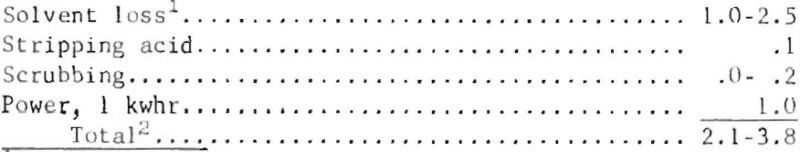
The- cost for producing cathode copper from the copper-acid electrolyte is estimated as equal to or less than the processing cost of cement copper in a typical operation.
Once, the economics of the solvent extraction of copper have been stabilized it is probable that commercial plants will be designed using processes similar to those used in modern uranium solvent extraction plants.
Smaller copper producers are more interested in the prospects of LIX-64 than are the major firms. The high cost of conventional smelting and electrorefining seems to be the major incentive toward attaining an economical solvent extraction process.
In October 1967, Ranchers Exploration and Development Corp. announced plans for a commercial installation comprising solvent extraction and electrowinning facilities. The 5,400-ton-per-year-capacity electrolytic section of this plant was to be installed near Miami, Ariz., at a cost of $3.5 million. The copper-acid feed solution to be processed by this facility originates from the solvent extraction of pregnant solutions produced by leaching ore loosened by ripping and placed on leaching sites.
Ion-Exchange Resins for Copper Recovery
Cationic carboxylic-type resins are the most promising with regard to selectivity and absorption capacity for stripping solutions partly decopperized by cementation. However, these resins have the disadvantages of being degraded mechanically and of being fouled by iron and aluminum ions.
Miscellaneous Recovery Processes
Demand for Nonmassive Copper
Sales of copper powder in the United States in 1965 totaled 31,200 short tons, including 1,870 tons of flake copper alloy and 936 tons of flake copper used as pigments and antifouling agents. Copper powders, selling for about 15 cents per pound more than the massive material, are generally made by atomization of molten metal, by the hydrogen reduction of copper oxide, and by precipitation from either acid or ammoniacal solutions. Two novel leaching processes described below were devised to supply some of the increasing demands for copper powder and copper oxide.
Banner’s Oxide Process
One new test facility was being constructed in mid-1966 by the Banner Mining Co. in Arizona at a cost of $500,000. This venture will provide operational data useful in the construction of commercial mills using Banner’s oxide process to recover copper from limestone-bearing copper ores. Ore, ground only to 10 mesh to prevent sliming, will be processed in a 60-foot counterflow drum using sodium hydroxide as one of several alkaline reagents. The amphoteric nature of copper allows it to react as either an electropositive or electronegative ion. As developed, Banner’s oxide process can produce refined copper either by direct chemical processing or by electrorefining.
Hydrogenation Process
Another new process employs the hydrogen reduction of a copper solution to produce copper powder or copper oxide. Since June 1966, Arizona Chemcopper Corp. has operated a $3,350,000 plant capable of producing 8,250 tons of contained copper annually in the form of copper oxide and of copper powders and briquets.
Copper for this plant originates in the heap leaching of oxide-copper rock. Cement copper, produced by the precipitation of copper from pregnant solutions by scrap iron, is leached in an oxygen-enriched, buffered, ammonium sulfate solution. The iron content in the leach solution is allowed to build up to 15 to 20 grams per liter to accelerate the leaching rate at 2.0 pH and 150° to 200° F. The resulting pregnant solution is filtered and then autoclaved at 425 psig and 250° to 280° F in the presence of hydrogen produced from reformed natural gas. Sulfuric acid is regenerated and copper powder is formed during the reduction step. Polyacrylic acid is added during autoclaving to minimize the plating of the copper powder.
Estimates indicate the following input per pound of copper equivalent produced in a 25-ton-per-day plant:
Electricity……………………………………..kwhr…………………0.3
Heat energy……………………………………Btu………………..10,000
Water………………………………………….gallon……………….1.0
H2SO4……………………………………….pound………………….0.15
Polyacrylic acid……………………………….do……………………0.01
Filter aid…………………………………………do…………………….0.005
(NH4)2SO4…………………………………….do……………………..0.05
The estimated total cost of these seven components is 1.6 to 1.9 cents
L-P-F Process and Dual Process
The L-P-F process converts copper in highly oxidized ores to metallic copper, by leaching and precipitation. The metallic copper responds to recovery by flotation in the form of cement copper.
The Dual process uses in-plant leaching followed by conventional flotation to recover copper from ores containing about equal weights of oxide copper and sulfide copper.
Crude ore, after crushing to minus ¾ inch, is ground to minus 3 mesh before separating into sands and slimes. The sands are leached with sulfuric acid to recover most of the oxide copper and a portion of the sulfide copper. The washed residual sands together with added lime are then ground to minus 200 mesh, after which more sulfide copper is recovered by conventional flotation. The tailings produced during the flotation of slimes for sulfide-copper recovery are leached in acid solution for additional oxide-copper recovery. Six to ten pounds of burned lime is added to each ton of leach sands processed in the alkaline sulfide circuit.
Oxide copper contained in the solutions from the separate leaching of sand and slimes is precipitated by sponge iron. This sponge iron is produced from a pyrite concentrate and recovered by flotation of tailings leaving the alkaline flotation circuit.
The precipitated copper and the sulfide-copper concentrates from the flotation circuit are mixed prior to smelting.
Bureau of Mines Process for Copper-Zinc Ores
Copper and zinc occurring together in an ore can now be separated using a solvent extraction process developed by the Bureau of Mines. The process eliminates costly smelter operations and provides an economic treatment of complex ores which now go unused.
Ore concentrate containing copper and zinc sulfide minerals is first calcined to remove sulfides. The oxidized material is leached with sulfuric acid to dissolve the metals. The resulting solution, after partial neutralization, is added to an organic solvent comprising 75 percent kerosene, 20 percent active di-2-ethylhexyl phosphoric acid, and 5 percent isodecyl alcohol modifier. During countercurrent extraction, the zinc is preferentially absorbed by the solvent while copper builds up in the aqueous phase. The metals are then recovered by conventional electrolysis.
Ion-Exchange Column
A process jointly developed by the Bureau of Mines and the Kennecott Copper Corp. uses a new type of ion exchange column to extract copper and uranium from pregnant solutions produced by waste dump leaching. Uranium concentrates produced by this process meet Government purity standards.
Other Processes for Precipitating Copper
General
Copper can be precipitated from solution as a sulfide, cyanide, thiocyanate, or hydroxide. However, these copper compounds are difficult to settle and filter and also must be redissolved before the copper can be reprecipitated by iron.
Cyanide
Five U.S. patents using cyanide-leaching techniques promise a break-through for producing a high-grade precipitated product from low-grade ores, copper-bearing materials, and solutions. The inventors of these patents claim low operating costs, small capital requirements, economical reagent recovery, and a method for treating refractory ores and waste.
Ammonia
Little copper is now produced by the ammoniacal leaching of native copper ore or tailings. Generally, after the removal of native copper by flotation, the remaining sand is leached in closed tanks by a solution containing free ammonia and cupric ammonium carbonate. The copper is recovered by distilling off the free ammonia from the pregnant solution, thereby decomposing the cupric ammonium carbonate to precipitate copper oxides and liberate carbon dioxide and ammonia. The carbon dioxide and ammonia are recovered in water Lo produce a leaching solution for reuse.
Electrowinning
The electrowinning, of copper-acid solutions produces marketable copper while regenerating sulfuric acid. Electrowinning equipment requires a high capital investment and is economically feasible only for solutions containing more than 10 to 25 grams copper per liter. At a lower copper concentration the presence of a significant amount of iron adversely affects the current efficiency owing to oxidation-reduction reactions. Electrowinning requires 8 to 10 times more power than does electrorefining, but the additional cost for power is nearly offset by lower labor costs. The electrowinning process requires a minimum of anode handling and scrap remelting because the insoluble antimonial-lead anodes often have a 10-year service life.
Commercial Installations
Ranchers Exploration and Development Corp. completed solvent extraction and electrowinning facilities at its Bluebird mine near Miami, Ariz., in 1967. This plant is the first of its kind to utilize the solvent extraction of copper from leach solutions followed by back extraction of the solvent by a sulfuric acid solution to produce an electrolyte from which marketable copper is precipitated by electrowinning.
For many years at Inspiration, Ariz., the copper concentration in the electrolyte processed by electrowinning has been maintained at the required level by adding a stronger solution made by the dissolution of cement copper in sulfuric acid.
CCS Electrolytic Cell
Pilot tests using the CCS cell, developed by the Continental Copper and Steel Industries, Inc., indicate that copper leach solutions from the field can be processed with a maximum current density of 30 to 35 amperes per square foot of cathode surface. This is a marked improvement over the 11- to 20-ampere current density employed in conventional copper electrowinning cell where a minimum copper concentration of 25 grams per liter is maintained in the electrolyte.
The CCS cell produces good copper deposition even though a large “bite” of the copper is removed from the electrolyte in each pass.
To date, figures suggest that the CCS cell will produce copper for about the same cost as the cementation route. Because of the continuing upswing in scrap-iron costs and the gradual downtrend in the cost of electricity, which may be even lower when more nuclear-generated electricity becomes available, the electrowinning process is expected to gain increased acceptance.
Outlook for Primary Copper from Leaching
Copper minerals in sub-mill-grade rock produced during current and future open-pit-mining activities will probably contribute most of the copper produced by out-of-plant leaching in the United States in the next 15 years, despite the optimism shown for the proposed in-situ leaching of an ore deposit fractured by a nuclear device. This statement is based on the substantial investments made since 1964 in additional dump-leaching facilities and cementation equipment utilizing the latest technology, and on a conservative prediction that dump leaching will produce 200,000 short tons per year of primary copper by 1970.
The recovery of copper from leach solutions using solvent extraction will attain diversified commercial importance for the in-plant leaching of complex ores where the volume of leach solution produced will be small compared with the volume produced during in-situ and dump leaching.
Dump-leaching practices expected to be commonly used in the future include-
- The use of mixtures of bitumens plus mill tailings to provide impervious bottoms under dumps.
- The improved fragmentation of materials placed on dumps Lo attain a greater copper recovery in a shorter time.
- The steady input of air during leaching through ducts placed in position while building the dump.
- The use of heated leach solutions.
- The treatment of barren solution, after copper precipitation, to remove most of the aluminum and part of the iron content before reuse of the solution for additional leaching.
Scrap iron will continue to be the principal precipitant for removing copper from leach solutions.
From a water-pollution standpoint, the deliberate leaching of copper-bearing sub-mill-grade materials and the recovery of copper from mine-drainage waters are fine examples of the effective removal of a metal that upsets the ecology of water courses. The Anaconda Co., at a cost exceeding $12 million in a 10-year period, has minimized the contamination of the Clark Fork River in Montana. Waters from the mining and smelting operations are processed to produce an effluent of neutral pH and containing only a trace of iron. The success of Anaconda’s pollution abatement plan is shown by the fact that fish are caught only a few miles downstream from the water treatment plant.
