Process Elements
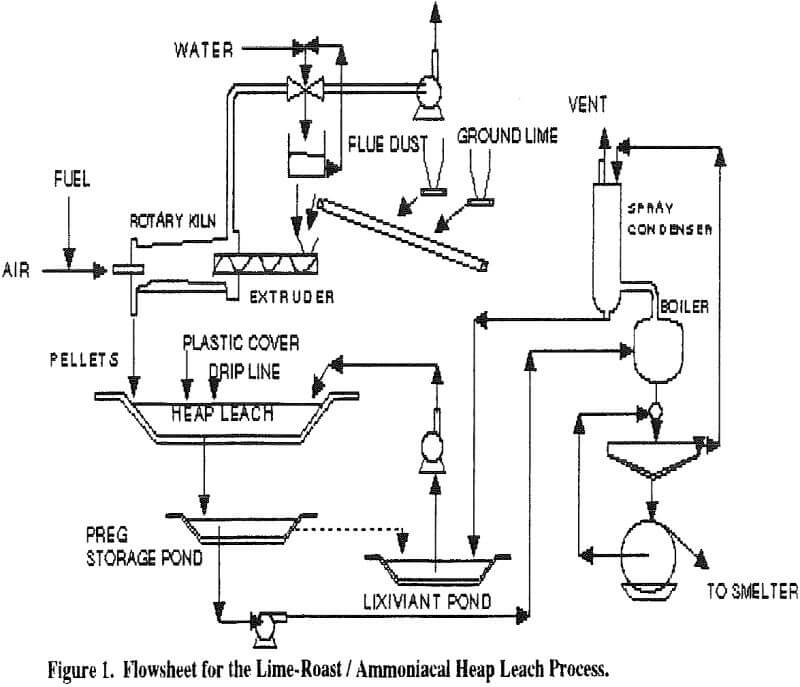
The process flow sheet is shown in Figure 1 and consists of the following steps:
- roasting a pelletized mixture of hydrated ground lime and flue dust to fix arsenic and sulfur; a venturi scrubber removes dust and any trace of escaping arsenic and sulfur.
- heap or vat leaching the roasted pellets with a solution of ammonia and an ammonium salt in a lined cell that is the final repository for the leached pellets.
- boiling the resulting copper pregnant liquor to vaporize ammonia and precipitate copper hydroxide, which is filtered and sold. The condensed ammonia and filtrate are combined and recycled as the leaching solution.
The lime-roast/ammoniacal leach process recovers copper while converting the flue dust to an environmentally acceptable product for permanent disposal in a lined repository. Arsenic is converted to calcium arsenite/arsenate, sulfur is converted to calcium sulfite/sulfate (anhydrite) and the heavy metals are entirely hydrolyzed and in a solid matrix at a pH above 10. Copper is dissolved during leaching because it is complexed with
ammonia. zinc, and some nickel if present, will also be dissolved but also precipitated and removed in the basic copper filter cake, iron and other heavy metals are not complexed or dissolved.
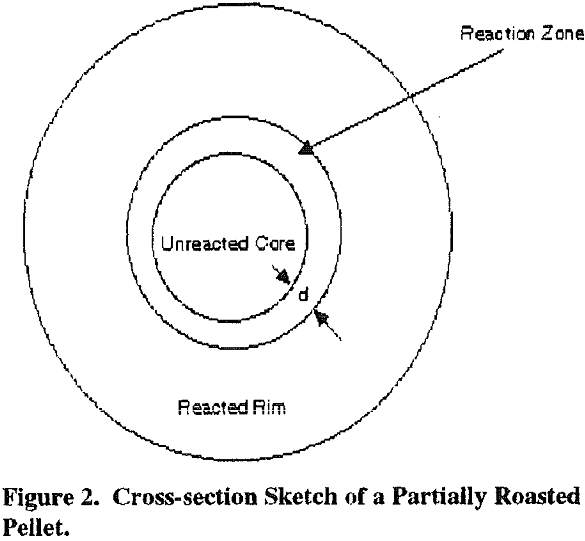
The normal oxidation reactions of residual sulfide minerals in the flue dust will occur during lime-roasting. Excessive temperatures must be avoided to prevent the formation of insoluble copper ferrites. A final roasting temperature in the vicinity of 600C has been found to be optimum in the previous studies, cited above, but good copper extractions have been achieved between 450C and 800C, and the optimum roasting temperature will vary with the specific roaster feed material.
Lime Roast Ammoniacal Heap Leaching
Arsenic is captured by what is believed to be the same mechanism as that for sulfur and in the same temperature range.
Leaching Copper with Ammoniacal Solutions
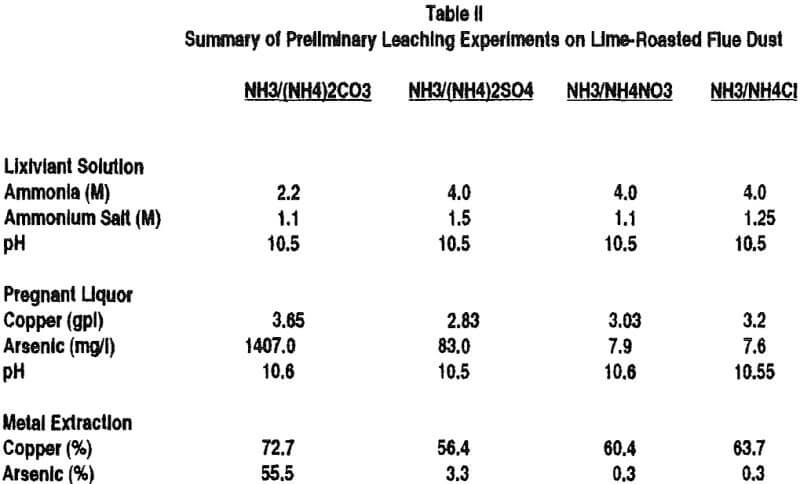
Various copper materials have been leached with ammine carbonate solutions (ammonia plus ammonium carbonate) and these include copper scrap, cement copper and various forms of oxidized copper such as roaster calcines. A Kansas City plant successfully leached copper scrap with ammine carbonate for many years.
It is necessary to have an ammonium salt present with ammonia so that the charge balancing anion for the cupric ammine complex [Cu(NH3)4] is not hydroxyl ion, which is what will result if only ammonia is used in the leaching solution. When only ammonia is used, the pH runs above 12 due to the hydroxyl anion and copper is precipitated as the hydroxide rather than dissolved as the ammine complex. With an ammonium salt present in sufficient quantity, the pH is lower, allowing copper to dissolve as the ammine complex.
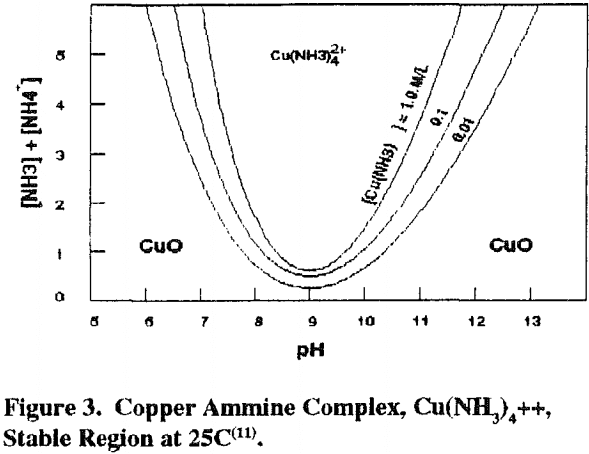
Ammonium salt is needed to provide anions other than hydroxl ion, to balance the cupric ammine complex charge without an excessive pH (hydroxl ion concentration) where the copper ammine complex cation is unstable.
Heap leaching has been extensively developed as a commercial process for the extraction of gold from permeable ore heaps using basic sodium cyanide solutions. The lixiviant solution is distributed evenly on the surface of the heap and solution percolates downward without flooding the heap. The ore particles are wetted by the percolating solutions which penetrate the ore particle pores by capillary action.
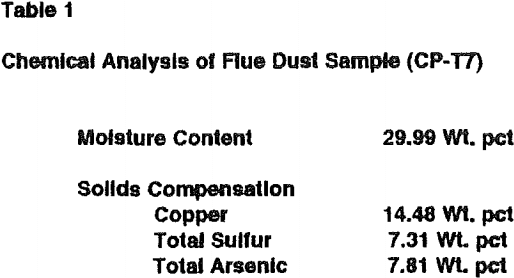
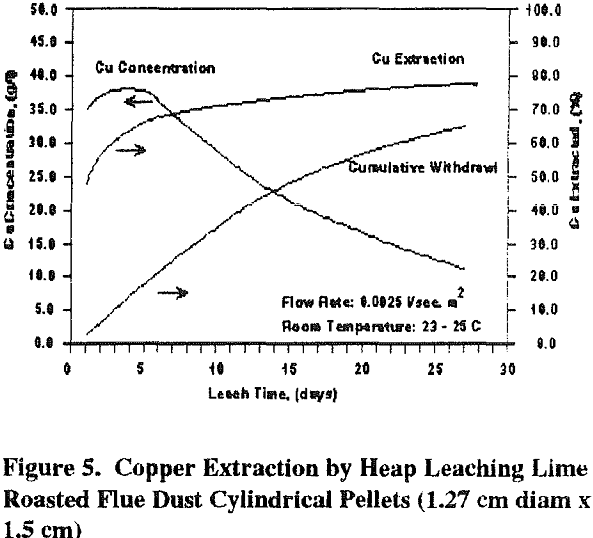
Simulated vat and heap leaching experiments were performed on cylindrical pellets (1.27 cm diam x 1.5 cm) of lime-roasted Anaconda flue dust. The amount of Ca(OH)2 used, 30 wt pct, was hyperstoichiometric with the total arsenic and sulfur content. Again, arsenic and sulfur in the tube furnace roaster flue gas were below detectable limits. The lime-roasted pellets had a specific gravity of 1.43 g/cc. Because of their uniform size, their apparent bulk density, in a heap or vat, is only 42 lbs/cu ft (0.67 g/cc).
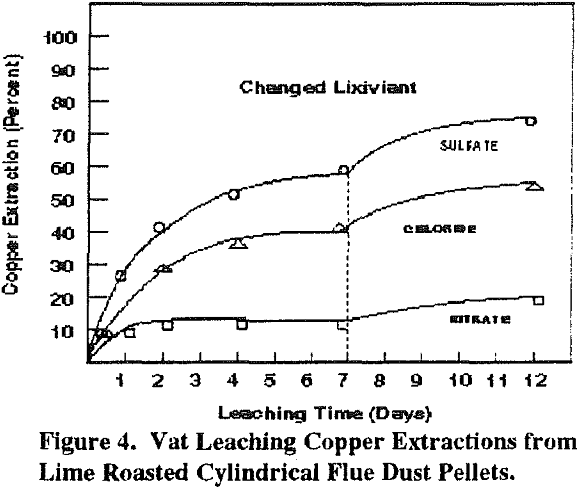
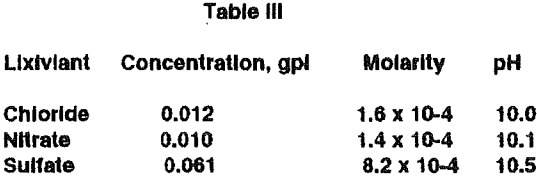
These results are consistent with our preliminary experiments with these lixiviants. Somewhat higher arsenic solubilities result with the sulfate lixiviant because of gypsum precipitation and a lowering of the calcium solubility. For the chloride and nitrate lixiviants, the arsenic molarity obtained agrees with thermochemical predictions. Under these environmentally optimum conditions with pH at about 10, calcium arsenate is extremely stable.
Ammonia vapor released from the boiler is cooled with circulating lixiviant, e.g. in a packed tower, and condensed to regenerate the lixiviant (leach) solution. With a large lixiviant storage pond in a reasonably cool climate circulating lixiviant through the condenser, additional cooling with a heat exchanger probably will not be required. The condenser is essentially a closed system, but minor venting of accumulated air or other noncondensible gases may be required.
