The electrolyte in-between the active electrodes must be purged with higher velocities to maintain a high concentration of the desirable copper ion in the liquid film adjacent to the cathode and the sulfate ion in the liquid film at the anode. This higher velocity also carries away from the cathode the undesirable impurities released from the anode as they are released into the electrolyte as filterable solids.
The Onahama refinery produced 5,300 metric tons of electrolytic copper per month in 1968 with a planned expansion to 12,000 tons per month. There are over 300 electrolytic cells in the tank house. About 540,000 gallons of copper sulfate electrolyte solution are recirculated through the cells at the rate of 5,000 gallons per minute. This means that all the electrolyte in the cells is replaced once every 80 minutes. About 400,000 gallons are in the cells and 150,000 gallons in a surge or service tank.
The copper sulfate concentration in the electrolyte is maintained at 49 gms per liter and the free sulfuric acid at 200 grams per liter. In addition to filtering the electrolyte at the rate of 2600 GFM through diatomaceous earth filter aid the electrolyte is also purified by dialysis. The electrolyte is maintained at 60°C (140°F) and a specific gravity of 1.2.
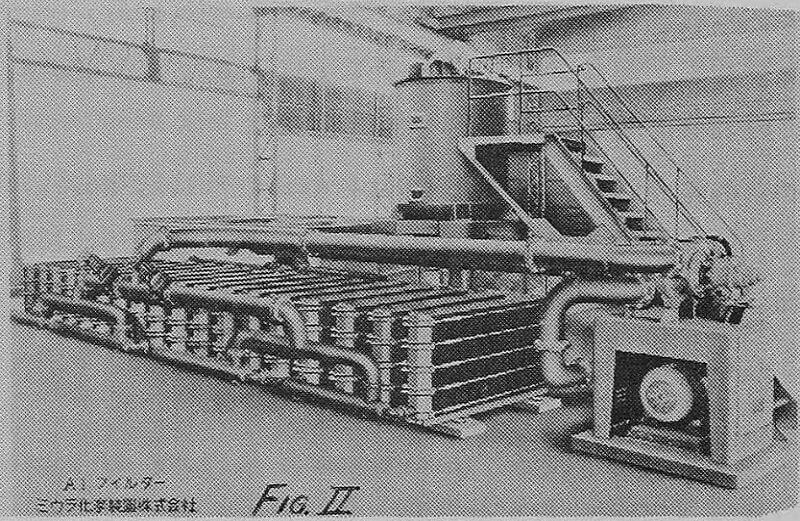
In the separation at Onahama the solution is of value and the filtered out slimes are of value. Therefore, a total separation and recovery is desired. The installed reversible filters f can only produce a wet cake or slurry discharge.
These were a 300 M²/hour system, a 600 M²/hour system, and a 3000 M²/hour system. The installation at the Onahama Refinery reported in this paper is the 600 M²/hour (2,600 GFM) installation.
There are two complete separate filtration systems. They occupy a room 40′ x 40′ by 10′ tall. Each can be operated independently of the other. They are cleaned out of phase so that one system is running while the other is being backwashed and precoated. Both draw electrolyte from a 150,000 gallon service or surge tank and return filtered electrolyte to the same tank. The main electrolyte re-circulating 5,000 GPM pump takes electrolyte from this service tank, pumps it through a heat exchanger to the cells, and back to the same service tank.
In the reversible filtration process the septum is backwashed with a fluid containing filterable plugging solids instead of a clear fluid. This results in a new cake forming on the opposite side of the filtering septum from the slide holding the cake to be discharged. This new cake forms only where there is flow through the septum and this would be those areas from which the old cake had been discharged from the down stream side.